Introduction -- FASTA -- BLAST -- Modular Domain -- Multiple Sequence Alignment -- PDB -- Summary
Introduction
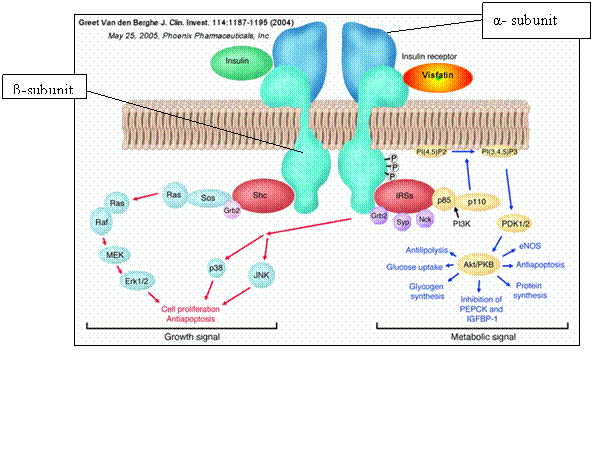
The insulin receptor is one of many proteins within the large class of protein kinases, and is closely related to the IRS-1 receptor. It can be observed at the surface of cells (most notably those of the liver, skeletal muscle and fat tissue), presenting themselves as dimer molecules, consisting of 2 unique subunit complexes, the α-and β-subunits, each with their own distinctive chemical properties, giving rise to the specific chemistry and functional behaviour of the receptor.
The α-subunit is responsible for binding hormones, whereas the tyrosine kinase domain and the ATP binding site lie resident in the β-subunit. The α-subunit remains exclusively outside the plasma membrane, whereas the β -subunit traverses across the membrane.
The gene encoding the sequence for the structural components of the insulin receptor can be identified on chromosome 19q. In the dimerized form, the insulin receptor is approximately 350kDa in size, with the slightly larger 2 α-subunits roughly 135kDa and the 2 β-subunits around 95kDa.
The insulin binds to the α-domain of the insulin receptor, inducing a conformational change in the 3-d structure of the receptor; prior to this process, the receptor resides in an ‘inactive’ form. This ligand binding of insulin stimulates the protein kinase (in this specific case the Tyrosine Kinase) activity within the intracellular domain in the 95kDa beta subunit, inducing a swift ‘auto-phosphorylation’ of particular tyrosine molecules on the β-subunits. This phosphorylation ignites a cascade response of phosphorylations from the cell. These signals are transduced to specific proteins downstream of the insulin receptor.
The IRS-1 receptor proteins are an example of a closely-related protein class which elicit a signal when activated by insulin (INSR1). The INSR-1 receptor allows proteins with an SH2 domain to occupy its phosphorylation sites. Once the INSR-1 has been phosphorylated by the INS- receptor, they in turn phosphorylate a series of kinase enzymes reliant upon PI3K (Protein kinase B for example) in a cascade series of intracellular reactions. These will eventually lead to regulation of enzymes which modulate glucose uptake and metabolism of proteins and lipids. Diabetes mellitus, type 1 and type 2 is strongly connected to insulin. It has been recently suggested that a reduced sensitivity of insulin by the insulin receptor may be a contributing factor to the development of type 2. In some specific contexts, antibodies may focus on the insulin receptor, producing insulin resistance of the receptor, leading to hyperglycaemia.
The insulin receptor also plays a role in activation of the ‘MAPK’ cascade pathway. Once insulin has bound to the insulin receptor, rendering it active, the tyrosine kinase activity of the receptor goes on to phosphorylate the ‘MAPK cascade kinase pathway’ via SHC, giving rise to stimulation in Ras signalling, affecting the rates of cell proliferation. Defects within this pathway are most notable for their roles in tumour progression.
Dephosphorylation of the insulin receptor results in the termination of activating phosphorylations on target signalling pathways.
REFERENCES
Oxford textbook of Medicine, David. A. Warrel, p320
Hormones, growth factors, and oncogenes: Enrique Pimentel, p87
Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein
Insulin receptor: evidence that it is a protein kinase
Direct activation of the phosphatidylinositol 3'-kinase by the insulin receptor
HYPERINSULINEMIC HYPOGLYCEMIA, FAMILIAL, 5; HHF5
The insulin receptor is essential for virus-induced tumorigenesis of Kaposi's sarcoma
vi: Hormones, growth factors, and oncogenes: Enrique Pimentel, p87
vii: Oxford textbook of Medicine, David. A. Warrel, p320
viii: Biochemistry: Pamela C. Champe et al; p309