Sub-study Patient Information Sheet
Study Title:
Legacies and Futures: Gestational Parents’ Experiences with Vulnerability and Resilience as it Influences Neonatal Health (student study – sub-study)
You are being invited to take part in a research sub-study as part of a PhD degree project. Before you decide whether or not to participate it is important for you to understand why the research is being done and what it will involve. Please take time to read the following information carefully. Talk to others about the study if you wish and ask us if there is anything that is not clear or if you would like more information. Take time to decide whether or not you wish to take part.
Research Project Summary:
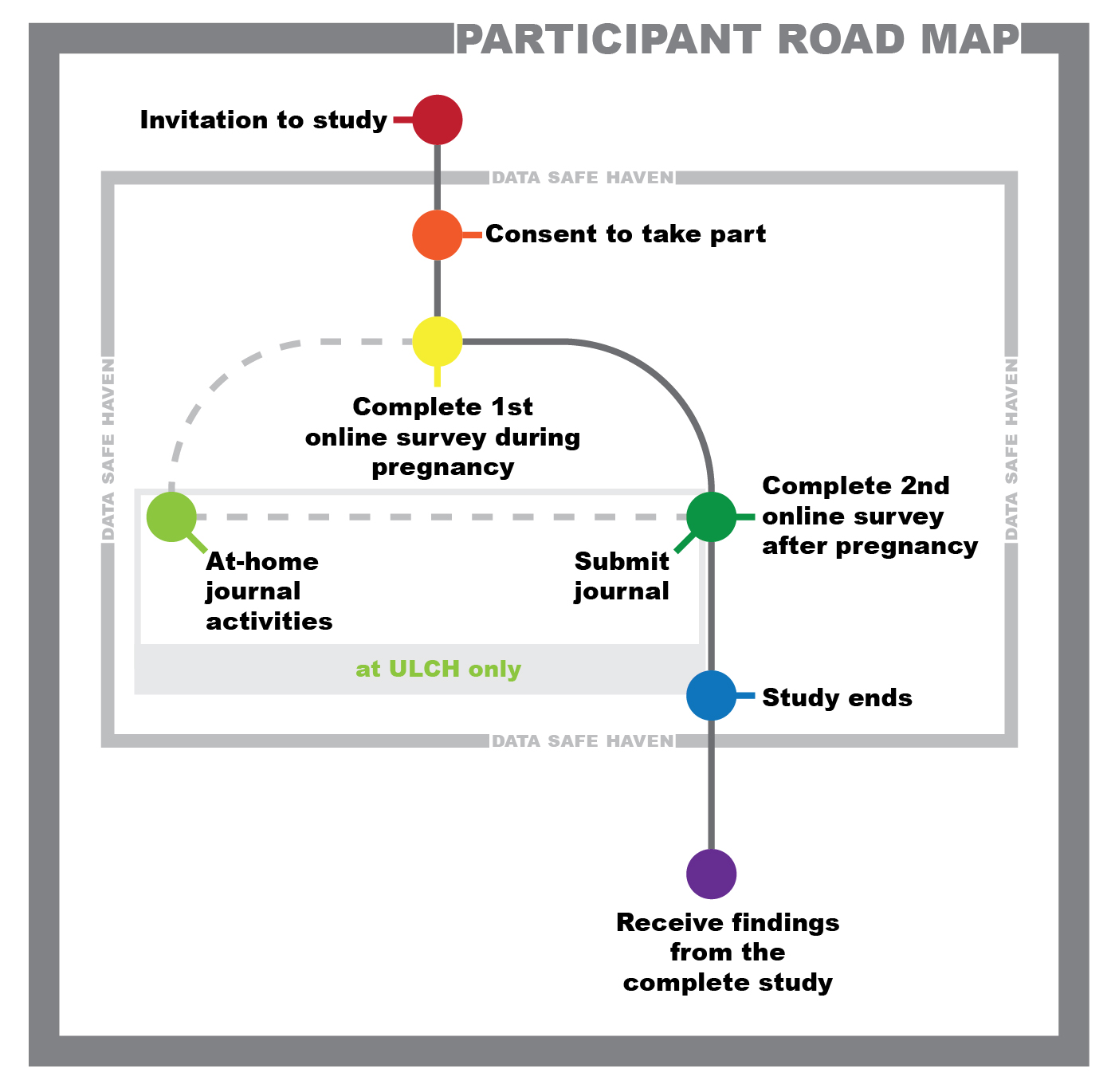
This project is focused on getting a better understanding of mental and physical health and well-being during pregnancy and postpartum recovery. While there have been similar studies in the past, this study provides a unique combination of survey results along with patient health records to help fill-in some of the missing gaps that can help improve care for all pregnant persons. We will be investigating similarities and differences between lesbian, gay, bisexual, queer, nonbinary, intersex, and/or transgender (LGBTQIA+) pregnant people and heterosexual pregnant people whose gender matches with their assigned sex at birth (cisgender).
Download the Sub-study Patient Information SheetThere is a set of directions that will come with the journal. Within the journal, there are prompts to guide you if you are stumped on what you want to include within the journal. Activities within the journal have no right or wrong way to be completed, so how they are completed are up to what information and approach you feel best reflects what you want to share with the study team.
If at any point you no longer feel comfortable participating in the sub-study, you are also able to leave the study by emailing (stnvkll@ucl.ac.uk).
As for the contents of the journal, you will be responsible for how you chose to keep your journal, so you will need to be aware of how you are storing the physical or digital journal and who may have access to it while you are completing the activities. We recommend selecting the version of the journal activity that you are best able to keep safely (e.g., a digital version kept with a password or a paper version that can be locked away).
The National Childbirth Trust
Website: https://www.nct.org.uk
Support line: 0300 330 0700
Gingerbread: Single parents, equal families
Website: https://www.gingerbread.org.uk
Helpline: 0808 802 0925
Mind
Website: https://www.mind.org.uk
Infoline: 0300 123 3393
Samaritans
Website: http://www.samaritans.org/
Helpline: 08457 90 90 90
Switchboard LGBT+ helpline
Website: https://switchboard.lgbt/
Helpline: 0300 330 0630
These support services are available to everyone who is pregnant. However, the general language used may centre on cisgender, heterosexual individuals.
If you wish to complain, or have any concerns about any aspect of the way you have been approached or treated by members of staff you may have experienced due to your participation in the research, National Health Service or UCL complaints mechanisms are available to you. Please ask your research doctor if you would like more information on this. In the unlikely event that you are harmed by taking part in this study, compensation may be available.
If you suspect that the harm is the result of the Sponsor’s (University College London) or the hospital's negligence, then you may be able to claim compensation. After discussing with your research doctor, please make the claim in writing to the David Frost (d.frost@ucl.ac.uk) who is the Chief Investigator for the research and is based at UCL. The Chief Investigator will then pass the claim to the Sponsor’s Insurers, via the Sponsor’s office. You may have to bear the costs of the legal action initially, and you should consult a lawyer about this.
If you have a concern about any aspect of this study, you should ask to speak to the researchers who will do their best to answer your questions, contact details are at the end of the document. If you remain unhappy and wish to complain formally, you can do this via the hospital’s Patient Advisory Liaison Service (PALS).
University College London Hospital
PALS can be accessed by visiting the office at either UCH Monday to Friday (closed all day Wednesday), or the NHNN Wednesday to Friday 9am – 4pm
Post:
PALS, Ground Floor Atrium, University College Hospital, 235 Euston Road, London NW1 2BU
Online contact form: http://www.uclh.nhs.uk/PandV/Helpandsupport/PALS/Pages/OnlineformforcontactingthePALSteam.aspx
Tel (main hospital): 02034473042
Tel (NHNN): 02034483237
Email: Uclh.pals@nhs.net
For more information, you can visit:
https://www.uclh.nhs.uk/PandV/Helpandsupport/PALS/Pages/Home.aspx
When contacting them, please quote the study number (IRAS: 264198).
The National Childbirth Trust
Website: https://www.nct.org.uk
Support line: 0300 330 0700
Gingerbread: Single parents, equal families
Website: https://www.gingerbread.org.uk
Helpline: 0808 802 0925
Mind
Website: https://www.mind.org.uk
Infoline: 0300 123 3393
Samaritans
Website: http://www.samaritans.org/
Helpline: 08457 90 90 90
Switchboard LGBT+ helpline
Website: https://switchboard.lgbt/
Helpline: 0300 330 0630
These support services are available to everyone who is pregnant. However, the general language used may centre on cisgender, heterosexual individuals.
Local Data Protection Privacy Notice
Notice:
The data controller for this project will be University College London (UCL). The UCL Data Protection Office provides oversight of UCL activities involving the processing of personal data, and can be contacted at data-protection@ucl.ac.uk. UCL’s Data Protection Officer can also be contacted at data-protection@ucl.ac.uk.
Your personal data will be processed for the purposes outlined in this notice.
The legal basis that would be used to process your personal data will be performance of a task in the public interest.
Your personal data will be processed so long as it is required for the research project. If we are able to anonymise or pseudonymise the personal data you provide we will undertake this, and will endeavour to minimise the processing of personal data wherever possible. Only authorised members of the research team will have access to your personal information. The anonymised data set will be kept for [10 years] after the end of the project, after which it will be reviewed to determine whether it would be appropriate to delete it.
If you are concerned about how your personal data is being processed, please contact UCL in the first instance at data-protection@ucl.ac.uk. If you remain unsatisfied, you may wish to contact the Information Commissioner’s Office (ICO). Contact details, and details of data subject rights, are available on the ICO website at: https://ico.org.uk/for-organisations/data-protection-reform/overview-of-the-gdpr/individuals-rights/
Your data will not be transferred to third parties. You will be given a copy of this information sheet and consent form for your records.
For more information, please read our privacy notice: https://www.ucl.ac.uk/legal-services/privacy/participants-health-and-care-research-privacy-notice
We also invite you to join the mailing list and to let you know when any presentation or publication come out of the study. Our Twitter will also be posting updates, find us @LegaciesFutures.
Further information and contact details
Kate Luxion
stnvkll@ucl.ac.uk
David Frost
d.frost@ucl.ac.uk
S. Melissa Whitten
melissawhitten@nhs.net
Thank you for taking the time to read this information sheet and for your participation in this study. Please keep this information for future reference.