Mechanism
Starting State
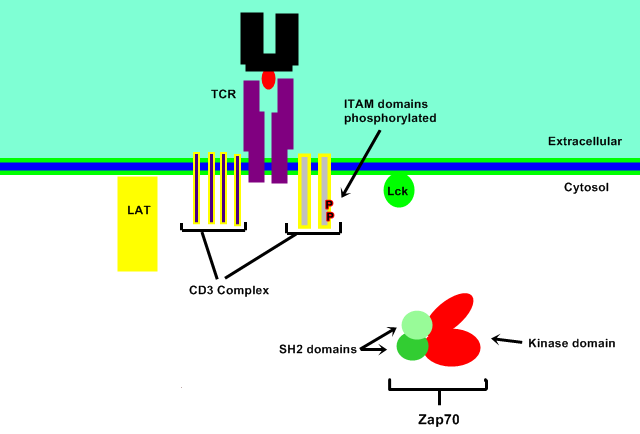
Membrane Localisation
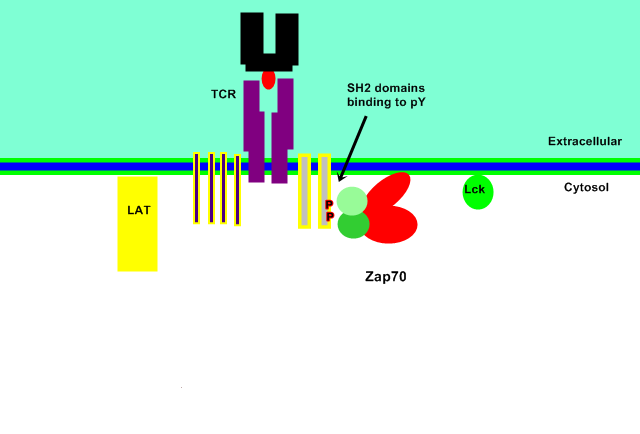
ZAP 70 is recruited and therefore localized to the plasma membrane region of the activated T-cell receptor (TCR). This occurs by the binding of the 2 tandem SH2 domains, located at the N-terminus of ZAP70, to the doubly phosphorylated ITAM motif within the CD247/CD3Z chains of the TCR and the phosphorylated TAM motif within RHOH.
Animation of ITAM and SH2 binding.
Release from Membrane
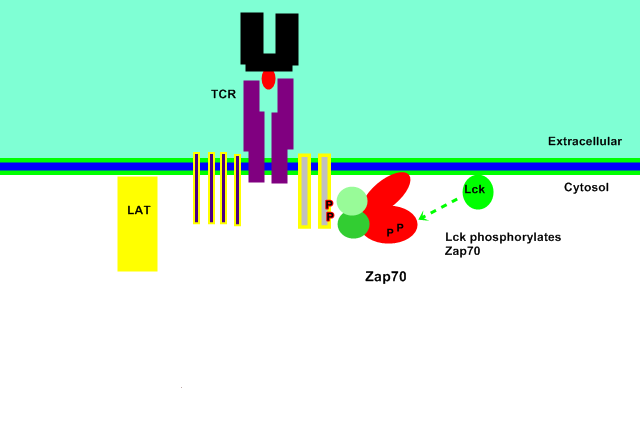
The bound ZAP70 is phosphorylated by Lck at Tyr-315 and Tyr-319 residues, subsequently causing a conformational change and its release from the plasma membrane as an active form.
Catalytic Site Uncovered
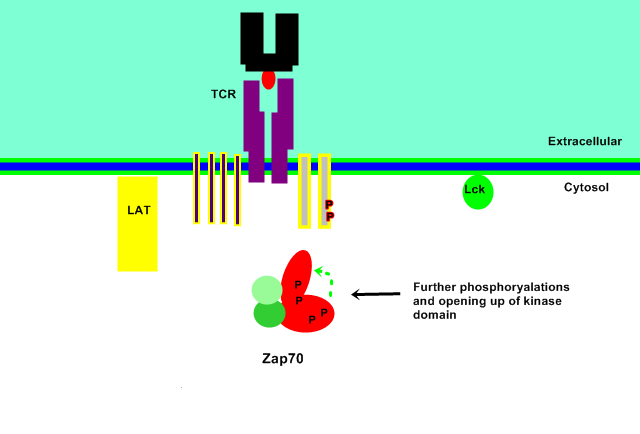
The active ZAP70 form is stabilised by further phosphorylation of the Tyr-492 and Tyr-493 residues within the tyrosine kinase domain. This causes the activation loop within the domain to pull away from the catalytic active site of the kinase, leaving it accessible to phosphorylate complimentary proteins.
LAT Binding
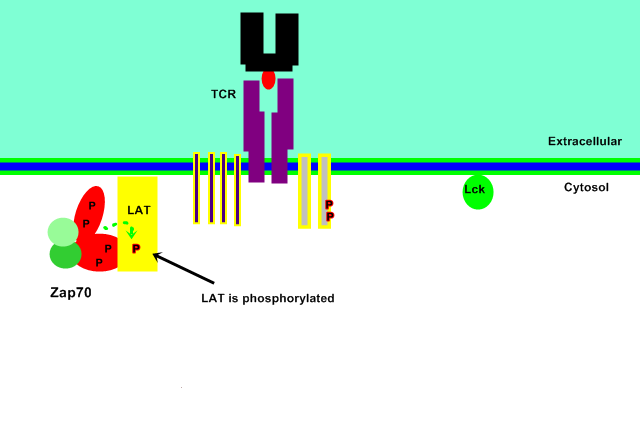
The active catalytic domain is then able to bind its substrate, an adaptor protein LAT (36-38kDa), and transfer the gamma phosphate from the ATP molecule within the kinase domain in a phosphorylation reaction to LAT, specifically targeting the tyrosine residues within the protein.
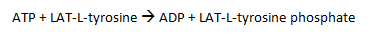
Signal Progression
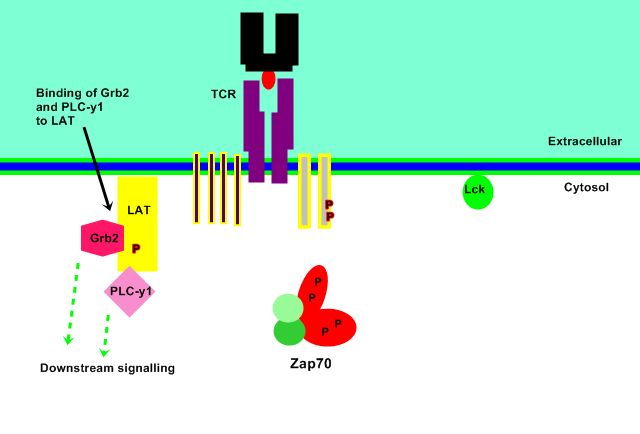
LAT then binds other crucial signalling molecules including Grb2 and PLC-γ1 to continue the immune response pathway downstream of ZAP70.