Inspired by my friend and collaborator Andrea Vezzoli's Website, here we give the history, background, and challenges behind our papers as they come out. The work is always more than the manuscript.
Why Black Phosphorous Forms Nanoribbons on Li/Ammonia Intercalation
Investigating the Mechanism of Phosphorene Nanoribbon Synthesis By Discharging Black Phosphorus Intercalation CompoundsNanoscale, 2024, 16, 1742-1750, 10.1039/D3NR05416K
The story of this Nanoscale paper is kind of one of failure. I was so convinced I had the perfect plan to show things worked as I had hoped, but it want to be. It is also the story of a hardworking MSc student (Qili Liu) and an absolute gem of a PhD student (Rebecca Shutt).
Since 2019, we have known you can make phosphorene nanoribbons (PNRs) from black phosphorus (bP) in what is ultimately a pretty simple 2-step process:
- You take solid bP crystals and lithium metal with an 8:1 stoichiometric ratio, and submerge in liquid ammonia overnight
- Take the resultant crystals and dissolve them in an amidic solvent
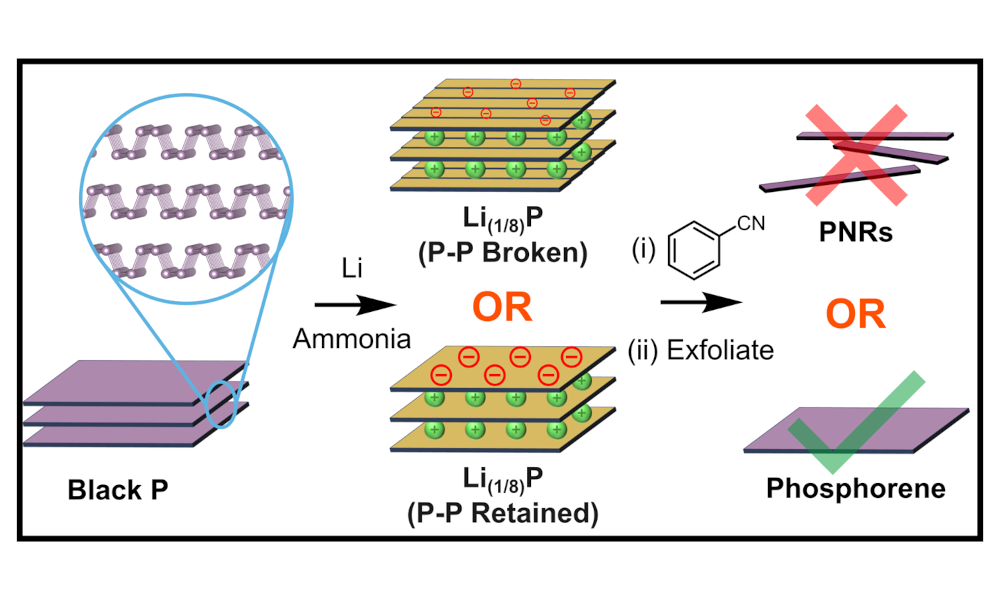
Long story short, I was convinced it was the first option, turning into ribbons when you first add the lithium. But how to prove it? A PhD student in the Howard group in UCL Physics, Becky, suggested stopping at the halfway stage and doing a tape exfoliation to separate the (alleged) ribbons. A good suggestion, and I did what any responsible PI would do and wrote an MSc project about this to offload the work and claim credit later: Qili soon joined and worked primarily under Becky’s tutelage.
The tape-exfoliation of the intercalated phosphorus failed immediately however. The weak van der Waals forces between 2D P-sheets had now been replaced by strong ionic bonds between anionic P-sheets and layers of lithium cations. Not all was lost however, and we had a plan. Or more accurately, a group in Erlangen had a reaction from years ago and I remembered it. If you add benzonitrile to lithium-intercalated-graphite, you can remove the lithium cation and the 2D-sheet-bound electron simultaneously. You can effectively undo the intercalation. As such, if there was ribbons made in Step 1, we could go back to our vdW bound sheets and the tape exfoliation plan was back on and I could see my ribbons! Even better, it would be a more air stable form of PNRs we could ship around for people to exfoliate at their leisure. Also, the benzonitrile goes a neat pink which is pretty aesthetically pleasing in a group where most of our chemistry is murky brown-orange or black.
And lo and behold, we deintercalated, we did the tape exfoliation and we saw…Drumroll... 2D phosphorene sheets.
Turns out the ribbons form in Step 2 by doing something with the solvent. And no, we still don’t know what that is.
Of course, this came with a bucket of characterisation as you might expect and astonishingly, Qili and Becky managed virtually an entire paper's worth in about 4 weeks, just before Covid lockdowns happened. The paper got pushed back several years, but in the interim was a bigger issue - how to get Qili her degree with an incomplete project and no access to a lab. The answer was DFT. I knew a bit, and with some help from Prof Furio Cora, we pivoted to understanding what happened to the P-P bonds upon Li intercalation. The real hero though is Becky who would have been perfectly in her right to focus on her own work, but she helped Qili by learning DFT alongside her each step of the way.
How and Why Nanotube Anions React
Crosslinked Single-walled Carbon Nanotube Aerogel Thin Films for Volatile Acid Sensors: Junction-mediated DetectionSubmitted, 2022
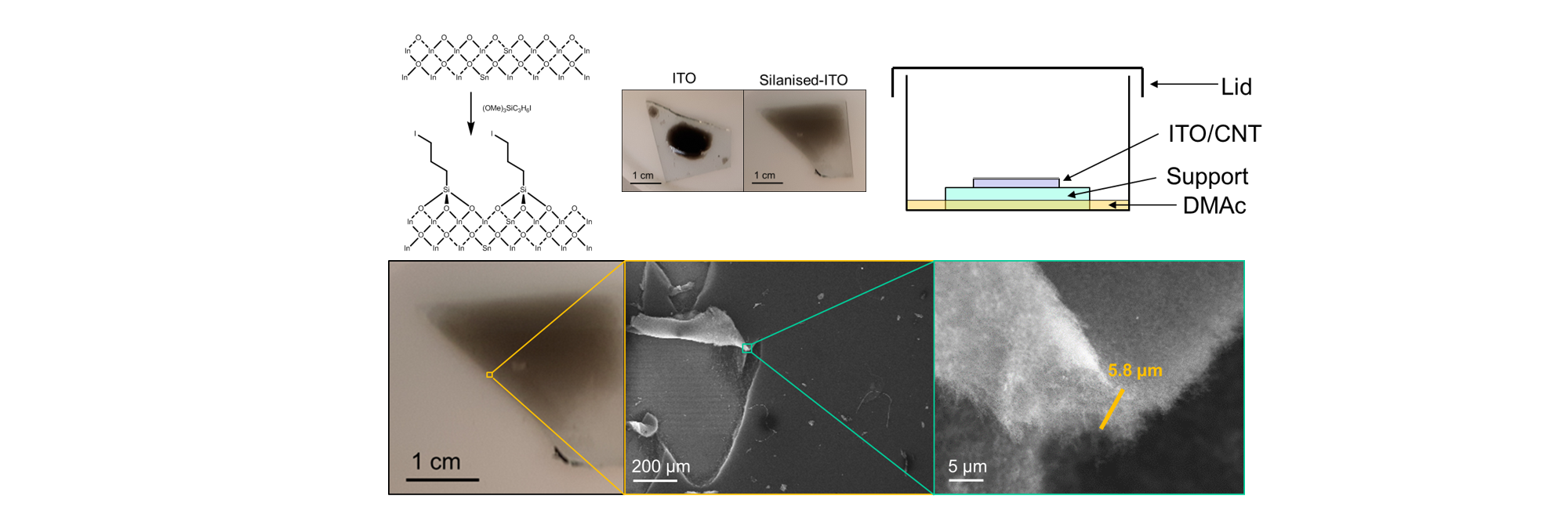
Science can take a really long time. The background to this paper isn’t the longest (I’m currently writing a paper based on data a collaborator took in 2009 and then forgot about), but it has been a long while. My contribution to this paper was mainly in November 2016 to be precise, about 6 years before submission. During my time at Imperial under Milo Shaffer, I had been roped into a collaboration with KIT where they wanted to make nanotube films infiltrated with a molecule for solar cell applications. The film was to be evenly a few nanometres thick, homogenous, and on ITO glass, which was a challenge. My approach was to make a thin aerogel, which could be infiltrated, then squished flat to give the desired film. The aerogel chemistry had been established previously by Martina De Marco, where you mix concentrated sodium nanotubide with di-iodobenzene in DMAc (i.e my favourite solvent) and leave for a day to make an organogel, which can be solvent exchanged and freeze-dried to give the aerogel. These were typically a few cm wide and freestanding however and I needed a few microns on glass. My first attempts to just make the gel-forming mixture on the glass failed however, as it didn’t wet onto the glass and it just made a blob-like droplet. It had failed 1 second in. This was all recorded in my group meeting slides where I compared my failure to Trump and Brexit… not that the next 6 years would make for better news.
 My solution was to silanise the glass with (OMe)3Si(C3H6)I which reacted with the ITO to give dangling iodoalkanes on the surface which would react to the nanotubide and make the surface more DMAc friendly. Now it wet beautifully - so it was solved, right? Well, no. Unfortunately to get the right thickness aerogel, I needed to add just 1.3 μL of gel-forming liquid per 1 cm2 of ITO slide and it dried after a minute or so, before the reaction completed, with drying effects destroying what little structure and porosity I had: there’s a reason you freeze-dry/supercritically-dry aerogels and not just dry-dry them. So how to stop it drying out? Cooling it would slow the drying but not stop the tiny amount leaving over 24h, so instead I exploited vapour pressure. If you saturate the atmosphere around the gel, then it will condense at the same rate it evaporates, so it wont dry out! After the reaction, I supercritically dried it with CO2, and voila! We had a 5.8 μm thick aerogel!
My solution was to silanise the glass with (OMe)3Si(C3H6)I which reacted with the ITO to give dangling iodoalkanes on the surface which would react to the nanotubide and make the surface more DMAc friendly. Now it wet beautifully - so it was solved, right? Well, no. Unfortunately to get the right thickness aerogel, I needed to add just 1.3 μL of gel-forming liquid per 1 cm2 of ITO slide and it dried after a minute or so, before the reaction completed, with drying effects destroying what little structure and porosity I had: there’s a reason you freeze-dry/supercritically-dry aerogels and not just dry-dry them. So how to stop it drying out? Cooling it would slow the drying but not stop the tiny amount leaving over 24h, so instead I exploited vapour pressure. If you saturate the atmosphere around the gel, then it will condense at the same rate it evaporates, so it wont dry out! After the reaction, I supercritically dried it with CO2, and voila! We had a 5.8 μm thick aerogel!
 So did the project work? Well, no. I can’t really even remember why. In all this mentioned above was a solid week of work, but I never worked on it again. However, we did have a promising new PhD student coming through called Dave Stringer, who was working to try and make gas sensors from carbon nanotubes. He was also using aerogels but instead of having benzene as the linker, he wanted to go more exotic and make things which would interact with molecules. See, nanotube sensors have long been a thing, but firstly their surface area is a bit rubbish (when not in aerogel form), and secondly, they aren’t very adaptable. By tweaking the chemistry of the CNT aerogel linker, his aim was to try and provide additional control over sensitivity, e.g. with amines that usually N-dope nanotubes reacting with acids to form nitrogen cations which wouldn’t. Change the doping and you (in theory) change the conductivity and hey presto you have sensing. I never got to see if this worked however, as a few months later I abandoned Imperial in favour of UCL. I still saw Stringer about (i.e. at the pub) and would happily chat about the nuances of playing with nanotubide, but it very much wasn’t my project. In the intervening years, the now-doctor Stringer was doing plenty of wonderful work in developing and improving these sensors and I won’t take any credit for all his endeavours. However, in 2022 I was sent a draft of a paper on aerogels and lo and behold, while the sensor work was more than I would have dreamed, underpinning it was two key tricks. 1) the substrate should be silanised with an alkyl iodide, and 2) it should be gelled in a saturated atmosphere of DMAc. No improving on perfection, I guess.
So did the project work? Well, no. I can’t really even remember why. In all this mentioned above was a solid week of work, but I never worked on it again. However, we did have a promising new PhD student coming through called Dave Stringer, who was working to try and make gas sensors from carbon nanotubes. He was also using aerogels but instead of having benzene as the linker, he wanted to go more exotic and make things which would interact with molecules. See, nanotube sensors have long been a thing, but firstly their surface area is a bit rubbish (when not in aerogel form), and secondly, they aren’t very adaptable. By tweaking the chemistry of the CNT aerogel linker, his aim was to try and provide additional control over sensitivity, e.g. with amines that usually N-dope nanotubes reacting with acids to form nitrogen cations which wouldn’t. Change the doping and you (in theory) change the conductivity and hey presto you have sensing. I never got to see if this worked however, as a few months later I abandoned Imperial in favour of UCL. I still saw Stringer about (i.e. at the pub) and would happily chat about the nuances of playing with nanotubide, but it very much wasn’t my project. In the intervening years, the now-doctor Stringer was doing plenty of wonderful work in developing and improving these sensors and I won’t take any credit for all his endeavours. However, in 2022 I was sent a draft of a paper on aerogels and lo and behold, while the sensor work was more than I would have dreamed, underpinning it was two key tricks. 1) the substrate should be silanised with an alkyl iodide, and 2) it should be gelled in a saturated atmosphere of DMAc. No improving on perfection, I guess.
How and Why Nanotube Anions React
Real-Time Mechanistic Study of Carbon Nanotube Anion Functionalisation Through Open Circuit VoltammetryChemical Science, 2019, 2019, 10, 3300-3306, 10.1039/C8SC04970J
Not a contemporary paper, but one harkening back to my PhD Plus (since rebranded the EPSRC Doctoral Prize Fellowship). Having submitted my proposal in August, I was told in November 2015 was being given a year’s worth of funding, to start in January 2016. I was overjoyed. The idea was simple: to apply the chemistries I knew and loved for carbon nanotubes to WS2 and BN nanotubes. Then it all fell apart. Firstly the company who made WS2 nanotubes emailed me saying they were now a company who previously made WS2 nanotubes, while a few weeks later, a group from Canada emphatically proved that the BNNT work was a good idea by doing it and publishing in ACS Nano.
Luckily, all this really meant was I had a year of funding to do something else, and I had an axe to grind with a very minor issue with something generally accepted in my field. See, to functionalise nanotubes, we negatively charge them, e.g. using sodium naphthalide, to give anions of nanotubide, dissolve them in a solvent, then add an alkyl halide, RX. A mechanism for this reaction was proposed years ago by Voiry: a scientist I have never met, with a fantastic portfolio of great work. The mechanism was a throwaway suggestion in his paper, but was broadly accepted to my bewilderment as it just didn’t reflect reality.
I wasn’t a fan.
So, I tried to follow the reaction myself and see if the other predictions held or we could get better insight into the behaviour. I had used electrochemistry while probing this effect during my PhD to try and quantify the remaining charge which the model preducted (it starts with a reduction of RX by CNT-, but as the reaction progresses, the reducing power of the nanotube will decrease, until it is negatively charged, but insufficently to reduce the RX). That previous work never saw the light of day as proof of this residual charge was proven in a very elegant JACS paper by Ferdinand Hof, who I have met and is a lovely guy. Didnt stop me being annoyed about being scooped by him. My big change now was to do the electrochemistry during the reduction and the functionalisaiton.
You can immediately see something weird here though. We are taking nanotubes hooked up to an electrode and negatively charging them and the charge becomes more negative. Which is good - we made anions. However, as soon as you add the alkyl halde, it becomes more negatively charged. And it does so for almost a full second - as seen in the zoomed in inset. The original Voiry mechanism showed the nanotube giving its electrons to the alkyl halide (i.e. becomeing less charged), and not only is this not happening, but adding the neutral alkyl halide is causeing the CNT anions to gain even more electrons. As it was unlikely to be RX reducing the CNT (RX + CNT[-] -> RX[+] + CNT[2-]), then what must be happening is that the condensed sodium counterion at the CNT surface are being displaced, giving a net more negative CNT. As this is longlived, it implies a complex is forming. After some steric trends and some DFT and more experiments, we showed this was a 2 electron transfer in a psudo-SN2, but you can read about all that in the paper. What really mattered for me was that I ended up obsessing over the nanotubide interface which led to my current research. And I showed that the old mechanism was wrong.
