
| Professor Lars Stixrude | 
|
| Home | Research | Teaching | Publications | CV |
|
|
|
|
|
|
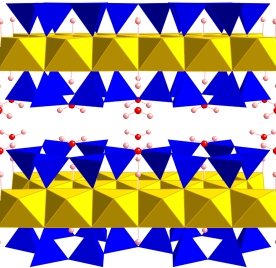
Hydrogen bonding in high pressure silicates and oxides
Nature of the hydrogen bond at high pressure Incorporation of hydrogen in nominally anhydrous phases Physics of high pressure hydrous phases How much water in Earth's Interior? The answer impacts our understanding of many aspects of Earth history from the evolution of the hydrosphere to mantle dynamics. We have been focusing on one aspect of the problem: what is the solubility of water in the mantle? Studies of hydrous phases, such as the 10 Angstrom phase, and nominally anhydrous phases, such as stishovite are showing us that ydrogen can be stably bound in mantle minerals to substantial depth and up to high temperatures. The solubility of water in silicate melts may be sufficiently large to substantially influence physical properties such as the density. The hydrogen bond may be very different at deep Earth conditions, and symmetric hydrogen bonding may be more common than it is at low pressure. Further Reading: Panero, W. R. and L. Stixrude, Hydrogen incorporation in stishovite at high pressure and symmetric hydrogen bonding in delta-AlOOH, Earth and Planetary Science Letters, 221, 421-431, 2004. PDF Fumagalli, P., L. Stixrude, S. Poli, and D. Snyder, The 10 Angstrom phase - A high pressure expandable sheet silicate stable during subduction of hydrated lithosphere, Earth and Planetary Science Letters, 186, 125-141, 2001 PDF |