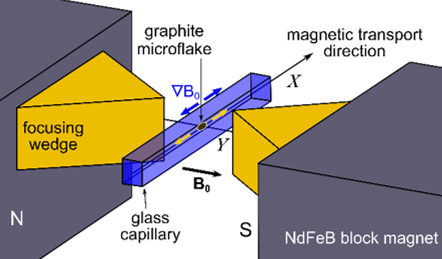
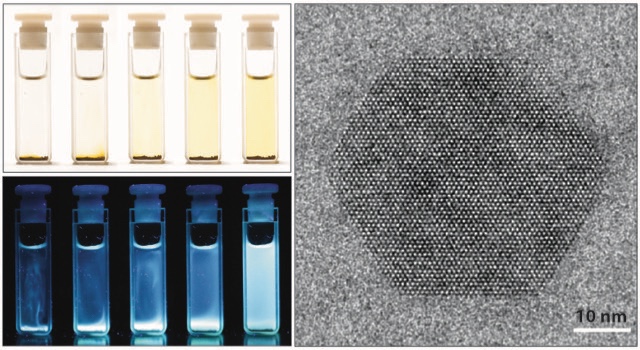
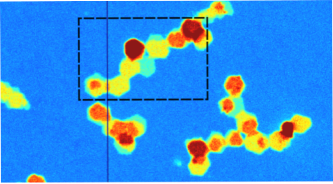
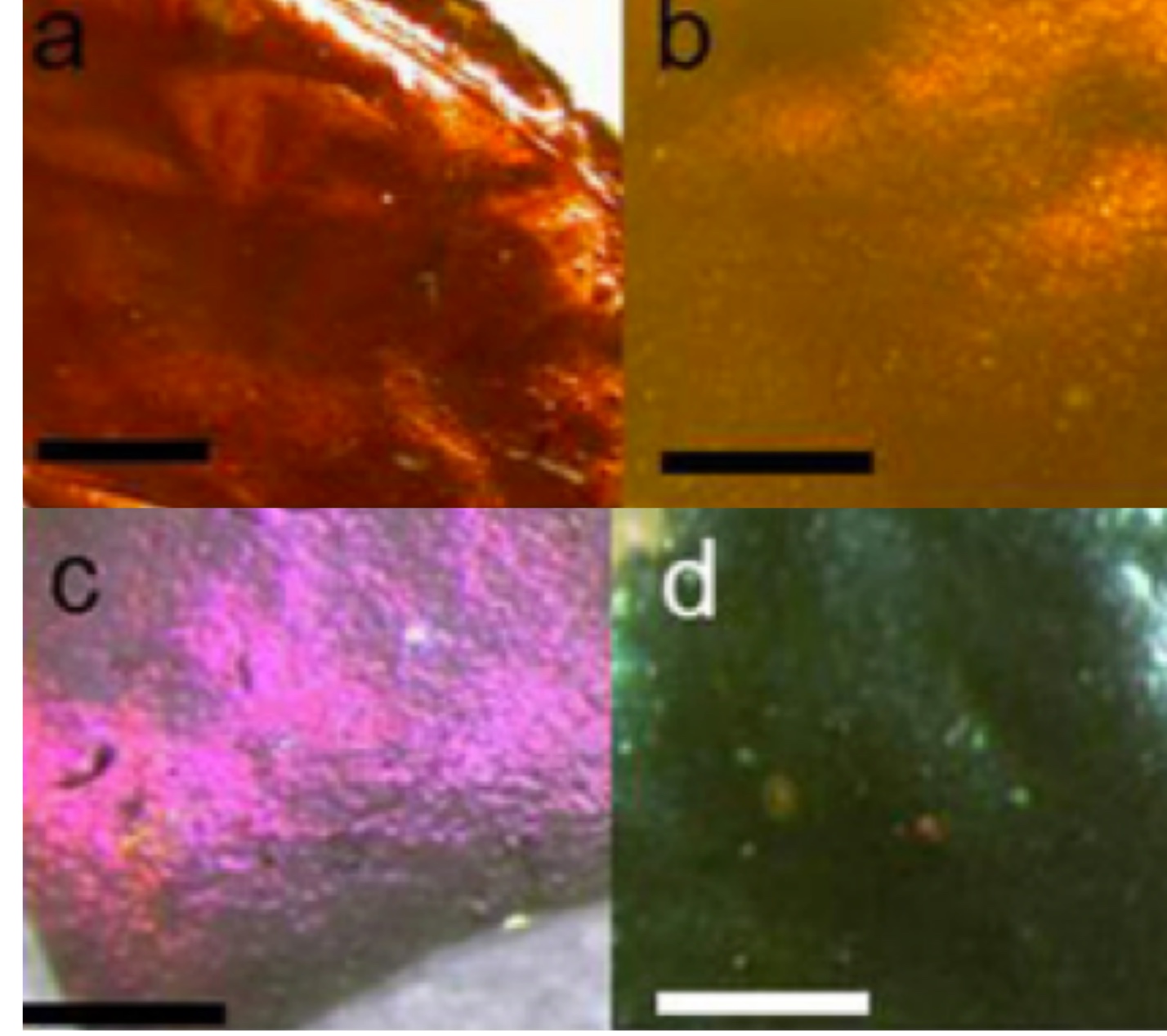
Welcome
Welcome to the research website of Chris Howard, Professor of Materials Physics in the Department of Physics & Astronomy at University College London. We create new materials that have desirable functional properties or that exhibit interesting emergent phenomena and we study these materials with a wide range of experimental techniques. Outputs range from nano-textured electrodes for battery and fuel cells to the discovery of exotic electronic groundstates. A particular expertise of the group is the ability to controllably dope (add charge carriers to) materials, especially nanomaterials and layered materials, via the addition of guest ions. The doping can drastically modify the material's properties (e.g turning a semiconductor into a metal) and we have also shown can enable the thermodynamic dissolution of the materials in liquids, for example, to form true solutions of 2-dimensional materials.
Recent Highlights
Tuning the interlayer spacing of graphene laminate films for efficient pore utilization towards compact capacitive energy storage
Li et al. Nature Energy (2020), 5, 160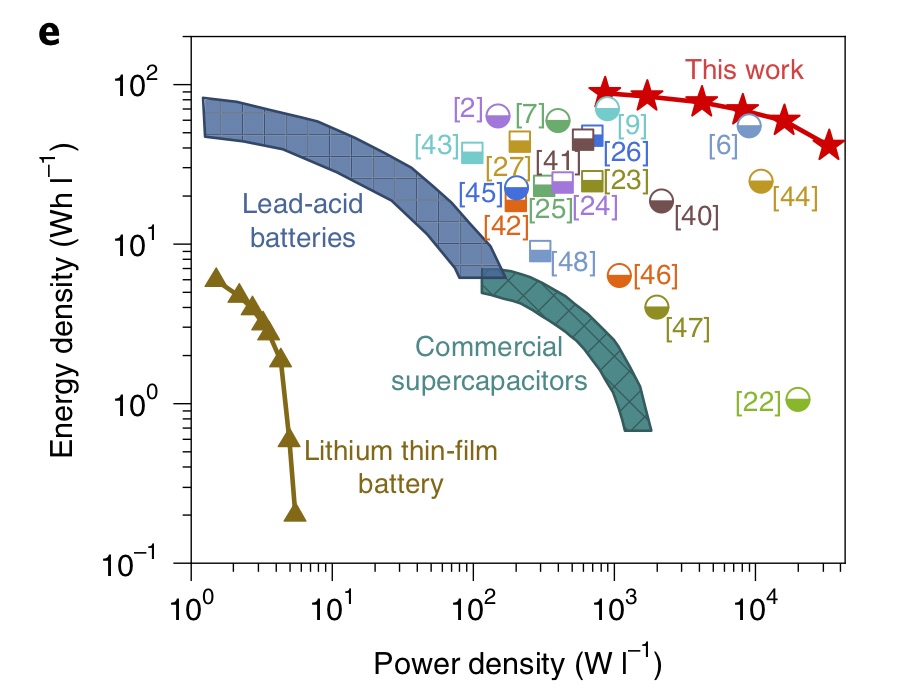
Supercapacitors have shown extraordinary promise for miniaturized electronics and electric vehicles, but are usually limited by electrodes with rather low volumetric performance, which is largely due to the inefficient utilization of pores in charge storage. Herein, we design a freestanding graphene laminate film electrode with highly efficient pore utilization for compact capacitive energy storage. The interlayer spacing of this film can be precisely adjusted, which enables a tunable porosity. By systematically tailoring the pore size for the electrolyte ions, pores are utilized optimally and thereby the volumetric capacitance is maximized. Consequently, the fabricated supercapacitor delivers a stack volumetric energy density of 88.1 Wh l-1 in an ionic liquid electrolyte, representing a critical breakthrough for optimizing the porosity towards compact energy storage. Moreover, the optimized film electrode is assembled into an ionogel-based, all-solid-state, flexible smart device with multiple optional outputs and superior stability, demonstrating enormous potential as a portable power supply in practical applications.
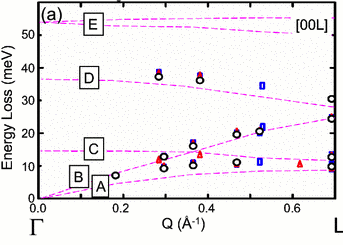
Production of phosphorene nanoribbons
Watts et al. Nature (2019), 568, 216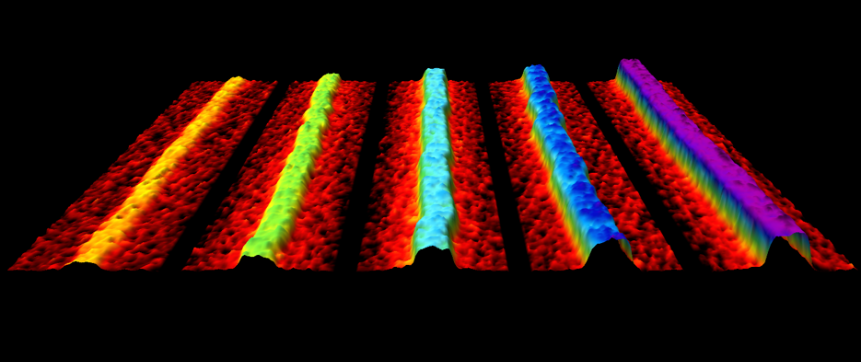
Over the previous five years there have been over 100 publications predicting that nanoribbons made from the 2-dimensional material phosphorene would have extraordinary properties. These properties could offer transformative advantages in applications ranging from fast-charging batteries to flexible thermoelectric devices to nanoelectronics. There have also been predictions of spin desnity waves, tunable magnetism, half-metallicity and topological states. However, until this paper no one had made discrete phosphorene nanoribbons (PNRs), and there was no obvious modification of methods for producing graphene nanoribbons to phosphorene. We describe a novel method for creating quantities of high-quality, individual PNRs by ionic scissoring of macroscopic black phosphorus crystals, and characterise the ribbons in detail. The ribbons have typical widths of 4-50 nm, predominantly single-layer thickness, measured lengths of up to 75 μm and aspect ratios of up to 1,000. The nanoribbons are atomically flat single crystals, aligned exclusively in the zigzag crystallographic orientation with remarkably uniform widths along their entire lengths, and are extremely flexible. Gallery of ribbons.