Research
Access to clean water
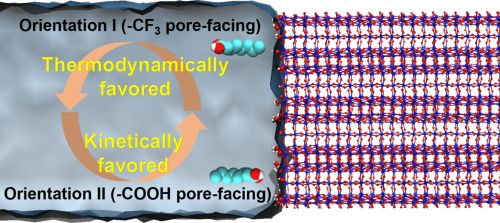
PFAS removal from water
The issue of contamination arising from per- and polyfluoroalkyl substances (PFAS), a class of synthetic chemicals containing at least one fully fluorinated carbon atom, has evolved into a significant environmental crisis. These PFAS compounds are notoriously resistant to degradation in nature due to the exceptional strength of carbon-fluorine bonds, earning them the moniker "forever chemicals". Our research in this field centers on the application of hydrophobic porous materials designed to specifically target the capture of PFAS at exceedingly low concentrations, addressing the most challenging aspect of the PFAS removal process.
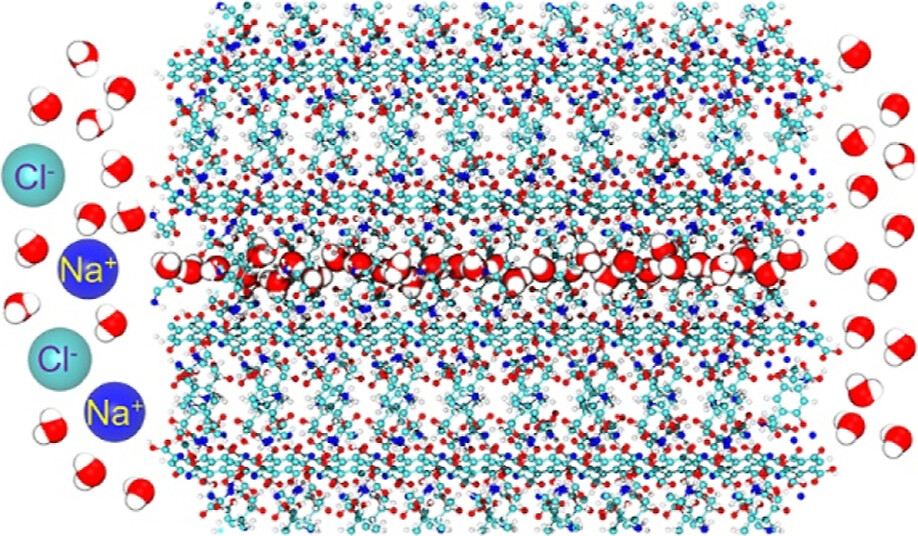
Design of biomimetic water purification membranes
Research into the creation and synthesis of artificial water nanochannels (AWCs) with a focus on achieving high flux and selectivity for water has gained considerable momentum. This heightened interest is driven by the envisioned applications of such AWCs in the field of water purification. In nature, aquaporin channel proteins play a pivotal role in facilitating water transport across the hydrophobic lipid bilayers of animal and plant cell membranes, a fundamental process vital for cell function. Drawing inspiration from aquaporin proteins and their mechanism for selectively transporting water, our research encompasses two key approaches: the computational design of porous materials engineered to mimic aquaporins, and the encapsulation of aquaporin proteins within solid-state nanopores.
CO2 capture, sequestration and utilization
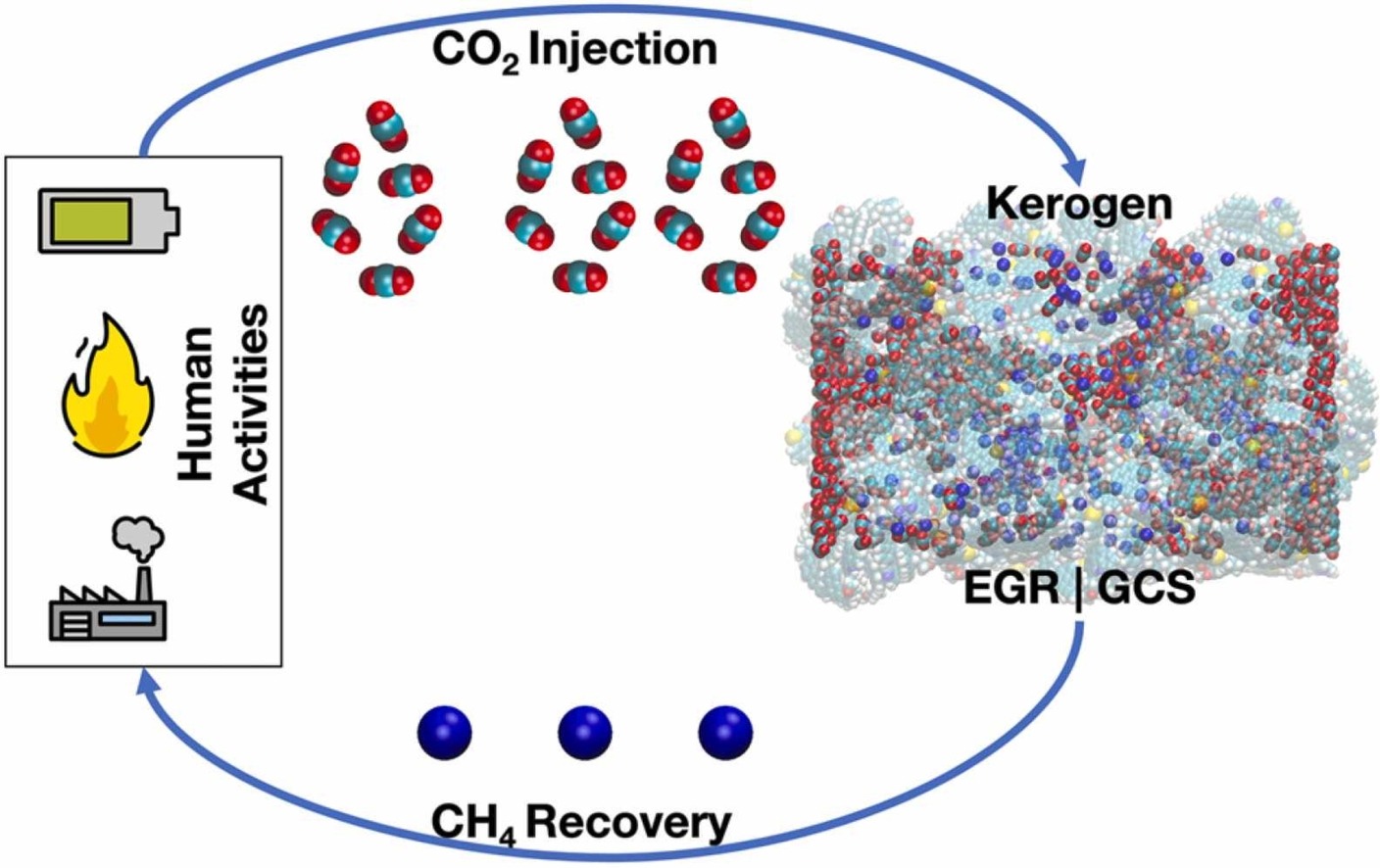
Our research efforts in this domain cover a broad spectrum of activities. We collaborate closely with experimental partners to evaluate the effectiveness of metal-organic framework (MOF) materials and analogous framework compounds for the purpose of capturing carbon dioxide (CO2) from wet flue gas. Our objective is to eliminate the dehydration process and achieve CO2 capture in a single stage, thereby mitigating the energy penalty associated with carbon capture.
After the capture of CO2, it is imperative to ensure its secure storage within geochemical formations or its utilization for alternative purposes. Our expertise in this domain is well-established, with a particular emphasis on the behaviour of sequestred CO2 in clay mineral layers as potential storage sites and harnessing captured CO2 for enhanced hydrocarbon recovery, particularly in nearly depleted shale reservoirs. Currently, our research is directed toward comprehending the behavior of sequestered CO2 within shale formations, specifically in the event of a potential leakage scenario.
Gas separation membranes
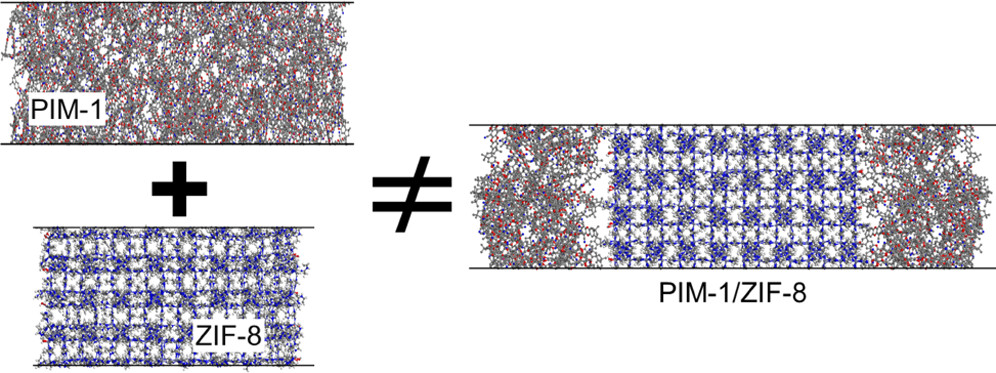
Thanks to the development of a novel non-equilibrium molecular dynamics approach in our research group, we have cultivated substantial expertise in modeling composite porous membranes for gas separation applications. Our collaboration with experimental partners is a consistent practice, enabling us to gain insights into the selective properties of these membranes. Presently, our research is centered on harnessing machine learning-assisted molecular modeling techniques to facilitate the development and discovery of materials capable of effectively separating CO2, nitrogen, and various other impurities from hydrogen .