|
|
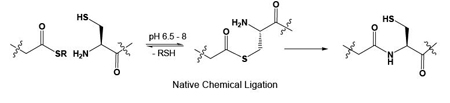
Native Chemical LigationSince its introduction Native Chemical Ligation (NCL) has facilitated the production of hundreds of proteins 1,2. NCL usually utilises two peptide components: a peptide C-terminal thioester and an N-terminal cysteine containing component. The two species combine to ultimately form a native peptide bond at the site of ligation. The reliability of the reaction and the mild conditions employed renders NCL as an excellent tool for building pure samples of modified proteins that are not readily isolable from natural sources and who’s activity cannot be readily dissected and deciphered using standard molecular biological tools such as site-directed mutagenesis.
In our laboratory, and in collaboration with other research groups we have introduced modifications such as unnatural amino acids, carbohydrates and phosphorylation into synthetic peptide fragments and assembled modified proteins by combining them in NCL reactions with larger protein fragments that are usually obtained form bacteria. This process (commonly known as Expressed Protein Ligation3,4, or Intein Mediated Protein Ligation5,6) has allowed modified proteins of much larger molecular weights to be routinely produced.
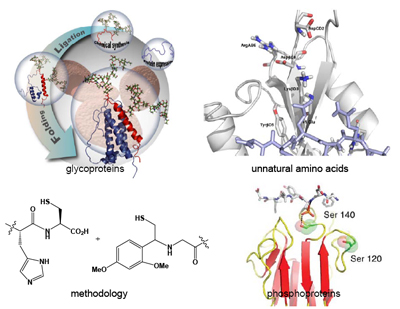
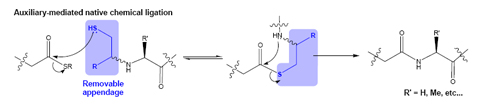
In collaboration with the Kajihara Group (Osaka, Japan) we have assembled a biologically active analogue of human Erythropoietin (EPO), an important glycoprotein therapeutic. In collaboration with the Smerdon Group at the MRC National Institute for Medical Research (NIMR) we have assembled a specifically phosphorylated variant of a human phosphoprotein involved in cell-cycle regulation.7 In collaboration with the Waksman group in UCL biochemistry/Birkeck Crystallography we have investigated the effect of unnatural amino acids (variants of lysine) on biomolecular recognition between SH2 domains and their cognate ligands. In addition to building biologically active molecules as potential therapeutics or research tools we attempt to contribute towards the development of NCL processes, in particular the development of mild or more generally applicable procedures for production of the thioester and N-terminal cysteine containing components for NCL which are compatible with more chemically fragile protein modifications such as glycosylation. It is often stated that NCL requires an N-terminal cysteine residue or a C-terminal thioester and with developments in NCL and NCL inspired methodologies these requirements have been largely overturned. Indeed NCL reactions have been shown between thioesters and components with N-terminal Cysteine, Serine8, Alanine9, Lysine10, Valine,11,12 and Phenylalanine13 after desulfurization of a β-amino thiol motif; Glycine, Alanine,14 and Glutamine15 (using thiol auxiliaries) and at several additional sites using analogous methods such as Sugar Assisted Ligation16. The thioester has also been replaced in several instances with β-keto acids (the decarboxylative amide ligation)17 and Cysteinylprolyl esters18. This burgeoning area is likely to present us with further breakthroughs in the near future. In our own Laboratory we have pursued an interest in acyl-transfer auxiliaries that allow us to assemble peptides and glycopeptide mimics in a cysteine-free manner19:
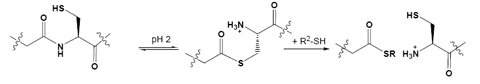
More recently we have become interested in a more unusual application of NCL where, at low pH, the reverse reaction can take place potentially providing access to peptide and protein fragments with C-terminal thioesters or N-terminal cysteines.20 Once optimised fully this process may give more straightforward access to components for NCL.
References: |