András Szabó
CV (pdf),
![]() ,
OrchID: 0000-0002-8924-038X
,
OrchID: 0000-0002-8924-038X
research
Collective cell migration
| Collective cell migration of the neural crest | UCL |
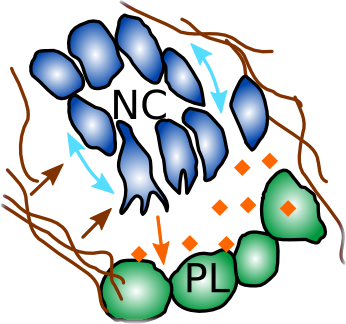 D
The neural crest is a transient cell population that migrates large distances collectively during development to contribute to most of the organs in the vertebrate body. The neural crest undergo EMT and acquire the cellular property of contact inhibition of locomotion (CIL). CIL is facilitated by a switch of E- to N-cadherin which allows the cells to repolarize and tear away from each other. During their migration, the neural crest is confined into streams by several extacellular molecules including the proteoglycan Versican, they undergo autocrine(C3a) and paracrine (Sdf1) chemotaxis, and perform CIL with each other and the neighbouring placodal cells that secrete Sdf1.
D
The neural crest is a transient cell population that migrates large distances collectively during development to contribute to most of the organs in the vertebrate body. The neural crest undergo EMT and acquire the cellular property of contact inhibition of locomotion (CIL). CIL is facilitated by a switch of E- to N-cadherin which allows the cells to repolarize and tear away from each other. During their migration, the neural crest is confined into streams by several extacellular molecules including the proteoglycan Versican, they undergo autocrine(C3a) and paracrine (Sdf1) chemotaxis, and perform CIL with each other and the neighbouring placodal cells that secrete Sdf1.
| Endothelial monolayers exhibit streaming behaviour in vitro | ELTE |
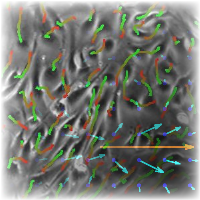 Endothelial cells keep their motility in vitro even for some time after growing into a confluent monolayer. Streams of several cell-widths form and disappear spontaneously, often creating cellular vortices and shear lines. We have characterised their streaming motion with a novel 2-dimensional velocity correlation function and gave a possible explanation to their behaviour. For this end, we introduced polarised cells to the cellular Potts model framework and gave a systematic analysis of the new model. The model readily describes the observations quantitatively.
Endothelial cells keep their motility in vitro even for some time after growing into a confluent monolayer. Streams of several cell-widths form and disappear spontaneously, often creating cellular vortices and shear lines. We have characterised their streaming motion with a novel 2-dimensional velocity correlation function and gave a possible explanation to their behaviour. For this end, we introduced polarised cells to the cellular Potts model framework and gave a systematic analysis of the new model. The model readily describes the observations quantitatively.
Morphogenesis in development
| Epiboly through radial intercalation driven by short-range chemotaxis | UCL |
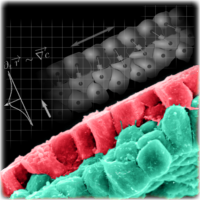 Epiboly is the large-scale morphogenetic process whereby a thick layer of tissue spreads and concomitantly thins in the perpendicular direction. During early development of the frog one hemisphere undergoes epiboly to cover the whole embryo. One of the essential drivers of this process is the radial intercalation of the inner cells towards the superficial cells that secrete a chemoattractant.
Epiboly is the large-scale morphogenetic process whereby a thick layer of tissue spreads and concomitantly thins in the perpendicular direction. During early development of the frog one hemisphere undergoes epiboly to cover the whole embryo. One of the essential drivers of this process is the radial intercalation of the inner cells towards the superficial cells that secrete a chemoattractant.
| Heart development and endothelial cell movement in the forming aorta | KUMC-ELTE |
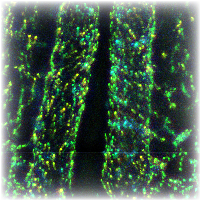
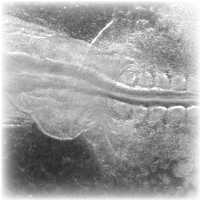 Under normal cicrumstances, endothelial cells in the forming aorta perform a spiraling motion towards the forming heart, before the onset of the circulation. With the aid of time-lapse fluorescent microscopy this motion is resolved in 3-dimensions. Cell-autonomous motility is devised from the observed cellular and extracellular matrix (fibrillin) motion.
Under normal cicrumstances, endothelial cells in the forming aorta perform a spiraling motion towards the forming heart, before the onset of the circulation. With the aid of time-lapse fluorescent microscopy this motion is resolved in 3-dimensions. Cell-autonomous motility is devised from the observed cellular and extracellular matrix (fibrillin) motion.
The forming heart of quail embryos is investigated with time-lapse fluorescent microscopy. Stages of development are introduced, and individual cell and tissue motion is analysed. The 3-dimensional velocity-field of the tracked parts is reconstructed, eventually yielding an overall picture of tissue and active cell motion within the heart region. A spiralling motion towards the heart is observable at the inflow tubes, very similar to the one apparent at the aortae.
Network formation of tissue cells
| Preferential attachment to elongated cells | ELTE |
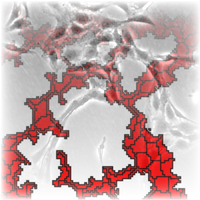 Several types of tissue cells are capable of forming networks similar to the primary vascular plexus, the predecessor of the vascular network in embryos. We have found that the cells move from seemingly random positions to connect into a network, even under circumstances that prevent the previously suggested mechanisms, such as chemotaxis. A possible mechanism for this phenomenon is the preferential attachment to elongated cells. Although the molecular mechanism behind the hypothesis remains unclear, computer simulations reveal that cellular networks can emerge. The hypothesis was implemented in the cellular Potts model as well as a simple model, where cells are represented by interacting points.
Several types of tissue cells are capable of forming networks similar to the primary vascular plexus, the predecessor of the vascular network in embryos. We have found that the cells move from seemingly random positions to connect into a network, even under circumstances that prevent the previously suggested mechanisms, such as chemotaxis. A possible mechanism for this phenomenon is the preferential attachment to elongated cells. Although the molecular mechanism behind the hypothesis remains unclear, computer simulations reveal that cellular networks can emerge. The hypothesis was implemented in the cellular Potts model as well as a simple model, where cells are represented by interacting points.
| Vascular sprouting | ELTE |
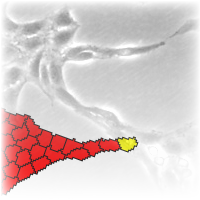 An important mechanism in remodeling existing networks is the formation of new sprouts from estabilished branches. Sprouts are initiated and pulled by tip cells, a specialized, actively moving sub-population. We argue, that the remainder, bulk of the population must also perform active motion, otherwise the sprouts would break up in the same fashion as water flowing from the tap breaks up into droplets. The assumption is supported by modeling. We used the cellular Potts model framework, with or without cells preferentially attached to elongated neighbours and a tip-cell with persistent active motility. The model is able to reproduce sprout initiation and growth. Cells without preferential attachment are unable to sustain the continuous growth of the sprout and thus the brach breaks, whereas if cells take an active part in the process, sprout growth is supported for an extended time and length.
An important mechanism in remodeling existing networks is the formation of new sprouts from estabilished branches. Sprouts are initiated and pulled by tip cells, a specialized, actively moving sub-population. We argue, that the remainder, bulk of the population must also perform active motion, otherwise the sprouts would break up in the same fashion as water flowing from the tap breaks up into droplets. The assumption is supported by modeling. We used the cellular Potts model framework, with or without cells preferentially attached to elongated neighbours and a tip-cell with persistent active motility. The model is able to reproduce sprout initiation and growth. Cells without preferential attachment are unable to sustain the continuous growth of the sprout and thus the brach breaks, whereas if cells take an active part in the process, sprout growth is supported for an extended time and length.
Computational tumor biology
| Evolutionary tumor growth modelling | NCSB-CWI |
Tumor behavior on a longer time scale is defined by the mutation and uncontrolled proliferation of malfunctioning cells. Several hypotheses exists on the actual time course and driving mechanisms of the development of such an aggregate. In the classical view (Nowell, 1976) the population goes through a well defined sequence of stages acquiring new phenotypes by gathering mutations of genes in a fixed sequence. More recent technology and research results enables us to look at the process in more detail, in terms of various phenotypes and their competition. Cell-based multi-level computational modelling is an especially useful tool in uncovering the dynamics and mechanisms underlying such complex systems.
| Simulation of active tumor invasion | ELTE |
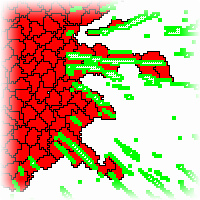 Active cell motion plays an important role in tumor invasion. By leaving the initial aggregate, cells are able to colonize new areas and thus spread the malignancy to larger areas. To understand the effect of a spatially organized microenvironment, we model active cell motion in ordered filaments of extracellular matrix. We show, that such an environment enhances persistent migration and therefore contributes to the dynamics. Furthermore, persistence in the cells' motion is also necessary to correctly describe invasion speed.
Active cell motion plays an important role in tumor invasion. By leaving the initial aggregate, cells are able to colonize new areas and thus spread the malignancy to larger areas. To understand the effect of a spatially organized microenvironment, we model active cell motion in ordered filaments of extracellular matrix. We show, that such an environment enhances persistent migration and therefore contributes to the dynamics. Furthermore, persistence in the cells' motion is also necessary to correctly describe invasion speed.
list of publications
- A. Shellard, A. Szabó, X. Trepat and R. Mayor Supracellular contraction at the rear of neural crest cell groups drives collective chemotaxis Science 362(6412):339-343 (2018)
- A. Roycroft, A. Szabó, I. Bahm, L. Daly, G. Charras, M. Parsons and R. Mayor Redistribution of Adhesive Forces through Src/FAK Drives Contact Inhibition of Locomotion in Neural Crest Dev Cell 45(5):565-579 (2018)
- A. Szabó and R.M.H. Merks Blood vessel tortuosity selects against evolution of aggressive tumor cells in confined tissue environments: A modeling approach. PLoS Comput Biol 13(7): e1005635 (2017)
- A. Szabó, M. Melchionda, G. Nastasi, M.L. Woods, S. Campo, R. Perris, R. Mayor In vivo confinement promotes collective migration of neural crest cells JCB 213(5):542-555 (2016)
- A. Szabó, I. Cobo, S. Omara, S. McLachlan, R. Keller, R. Mayor The Molecular Basis of Radial Intercalation during Tissue Spreading in Early Development Dev Cell 37(3):213-225 (2016)
- A. Szabó, R. Mayor Modelling collective cell migration of neural crest Curr Op Cell Biol 42:22-28 (2016)
- E. Scarpa, A. Szabó, A. Bibonne, E. Theveneau, M. Parsons, and R. Mayor Cadherin Switch during EMT in Neural Crest Cells Leads to Contact Inhibition of Locomotion via Repolarization of Forces Dev Cell 34(4):421 (2015)
- A. Szabó, R. Mayor Cell traction in collective cell migration and morphogenesis: The chase and run mechanism Cell Adhesion & Migration 9:380 (2015)
- D. Palachanis, A. Szabó, and R.M.H. Merks Particle-based simulation of ellipse-shaped particle aggregation as a model for vascular network formation Comp. Part. Mech 2(4):371 (2015)
- A. Czirók, K. Varga, E. Méhes, and A. Szabó Collective cell streams in epithelial monolayers depend on cell adhesion New J. of Phys. 15 (2013) 075006
- A. Szabó, R.M.H. Merks Cellular Potts modeling of tumor growth, tumor invasion and tumor evolution Frontiers in Oncology 3:87 (2013)
- A. K. Lagendijk, A. Szabó, R.M.H. Merks, J. Bakkers Hyaluronan: A critical regulator of endothelial-to-mesenchymal transition during cardiac valve formation Trends in Cardiovascular Medicine (2013)
- A. Szabo, K. Varga, T. Garay, B. Hegedus and A. Czirok. Invasion from a cell aggregate -- the roles of active cell motion and mechanical equilibrium Phys. Biol. 9 (2012) 016010
- A. Aleksandrova, A. Czirok, A. Szabo, M. B. Filla, M. J. Hossain, P. F. Whelan, R. Lansford, B. J. Rongish. Convective tissue movements play a major role in avian endocardial morphogenesis Developmental Biology (2012) 363(2):348
- A. Szabo, P.A. Rupp, B.J. Rongish, C.D. Little, A. Czirok. Extracellular matrix fluctuations during early embryogenesis Phys. Biol. 8 (2011) 045006
- A. Szabo, R. Unnep, E. Mehes, W. Twal, S. Argraves and A. Czirok. Collective cell motion in endothelial monolayers Phys. Biol. 7 046007, 2010.
- A. Szabo, A. Czirok. The role of cell-cell adhesion in the formation of multicellular sprouts. Math Model Nat Phenom 5(1):106, 2010
- A. Szabo, E. Mehes, E. Kosa, A. Czirok Multicellular sprouting in vitro. Biophys J. 95(6):27022710, 2008.
- A. Czirok, E. A. Zamir, A. Szabo, and C. D. Little Multicellular sprouting during vasculogenesis. Curr Top Dev Biol, 81:269289, 2008.
- A. Szabo, E. D. Perryn, and A. Czirok Network formation of tissue cells via preferential attraction to elongated structures. Phys Rev Lett, 98(3):038102, 2007.
- A. Szabó: Empirical and computational study of vasculogenesis. PhD dissertation (pdf)
links
Institutes
- UCL: University College London, Research Department of Cell and Developmental Biology, Mayor lab
- NCSB: Netherlands Consortium for Systems Biology, Netherlands Institute for Systems Biology (NISB), Biomodeling & Biosystems Analysis group
- CWI: Center for Mathematics and Computer Science, Biomodeling & Biosystems Analysis group
- KUMC: Univeristy of Kansas Medical Center, Dept. of Anatomy and Cell Biology, collaboration with: András Czirók, Charles D. Little, Brenda Rongish
- ELTE: Eötvös University, Budapest, Dept. of Biological Physics, Cell motility group of András Czirók
Software
- Tissue Simulation Toolkit: a Potts model framework @ sourceforge
- CompuCell3D: a Potts model framework
- NCSB SEDML Repository: projects in computational biology
Other information
- Masters thesis projects in the CWI Life Sciences group
- A short summary of my PhD thesis is published in the ESMBT's Communications (download page, pdf).