| Research |
| Teaching |
| Publications |
| DensEl |
| ParaDens |
| Conquest |
| Links |
| Home |
(If you're looking for supplementary data for Phys. Rev. B 67, 115341 (2003), you can find it for diffusion of the first hydrogen and second hydrogen; otherwise, please read on to find out more about the context of this fascinating question.)
Gas-source growth of Si(001)
Gas-source growth of Si(001) is of great interest, both technologically and scientifically: in particular, the hydrogen which remains on the surface (due to the silane (SiH4) or disilane (Si2H6) sources typically used) acts as a surfactant, promoting flatter growth and, if Ge is added to the growing surface, delaying the onset of strain effects (which I'm also investigating here). We have explored the whole range of gas-source growth and mapped out the pathway from initial adsorption to growth of islands (most of this work is described in Surf. Sci. 394, 79 (1997) and Surf. Sci. 394, 91 (1997)). One question that eluded us was how clean silicon dimers were formed: we knew that hydrogenated dimers formed easily (illustrated below in an STM image of the Si(001) surface exposed to a small dose of disilane taken by James Owen: the non-rotated hydrogenated dimers are marked as NRMH) but not how they then transformed into clean dimers at temperatures well below the H desorption temperature (at 450K, where H starts to desorb at 790K).
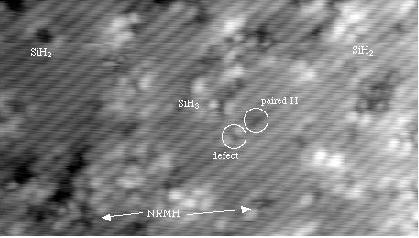
An STM image of Si(001) exposed to disilane.
Various features are marked: disilane fragments
(SiH2 and SiH3); paired hydrogens and
defects; and a hydrogenated dimer (parallel to the substrate
dimers) notated as NRMH.
The Problem
We start with this structure (only the top two layers of Si
are shown for clarity): 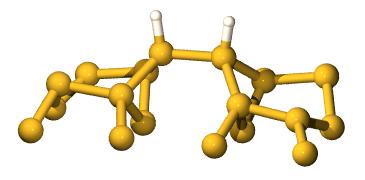
and end with this structure (where the H are now on the
substrate dimers): 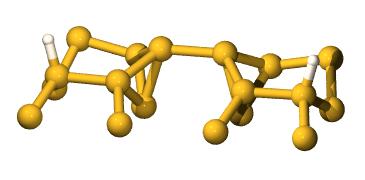
The problem is
that the H can't just diffuse off the dimer, as the barrier is
prohibitively large.
The Key State
It turns out that the diffusion proceeds via a meta-stable
intermediate state, illustrated here: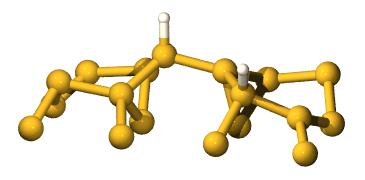
This meta-stable state is
discussed in more detail here, and you can also
find the details for the diffusion of the first hydrogen and second hydrogen off
the hydrogenated ad-dimer. The calculated barriers agree
extremely well with the temperatures at which the
dehydrogenation is observed to occur.
This research has been submitted to Physical Review B and is available as a preprint in either gzipped postscript or PDF