Research
This page is intended to be a broadly accessible discussion of some of the major themes raised by the research which I have led and overseen. To date there have been four key themes, although many studies I have worked on stand alone, without falling directly into any of these categories. One theme, which was the primary focus of my PhD thesis, is the mainly technical problem of repeatably finding specific white matter structures in the brain, which needs to be solved to facilitate other research. The second is a more practical question, of how the brain’s “wiring” changes as the body develops and gets older. I have also been interested in how to study the brain network as a whole, combining different sources of information to get a more complete picture than we could do using any one source alone. Most recently, I have been interested in bilingualism and the developing brain, not least because my children have parents with different native languages.
These are discussed further below, with links to relevant published work from my group. Of course, these are active research areas under investigation by many groups, and fuller discussion of the relevant scientific literature can be found in each cited paper. I also teach in areas related to many of these themes.
Finding specific brain structures
Diffusion magnetic resonance imaging (MRI) is a rich source of information about brain structure, particularly the white matter which forms the brain’s “wiring”, connecting areas of grey matter together. This medical imaging technology is available for the MRI scanners installed in many hospitals, but its full clinical potential has probably not been realised yet, partly because it requires complex image processing and specialised expertise to produce reliable results. Identifying the exact pathway of a specific white matter “tract” is hard to do consistently from one brain to the next, not least because these structures are three-dimensional and often twist and turn considerably. However, each brain generally contains the same set of major tracts, and each tract generally connects the same grey matter regions via essentially the same route.
Taking advantage of this similarity in tract shape across individuals, we developed a computational method which uses a “reference tract” to provide an archetypal trajectory for each structure. The tract of interest is then identified in each subject by using an algorithm to find the trajectory with the most similar shape to this reference. This approach, called “neighbourhood tractography”, was developed into a semi-automated algorithm, refined, and later extended as a means to “tidy up” the virtual representations of each tract after they have been identified. Implementations of the various algorithms are freely available in the TractoR software package.
Two main limitations remained with the neighbourhood tractography approach: the full tract extent is usually not included and the process takes some time. Motivated by applications in neurosurgery, where time is very limited and knowing the full extend of the tract is important, we explored a new approach, ultimately called “tractfinder”. This avoids conventional tractography entirely, in favour of a simpler combination of an atlas and the tract orientation information available from the patient’s scan. In combination with a distortion method to handle space-occupying brain lesions such as tumours, this proved a robust and effective approach.
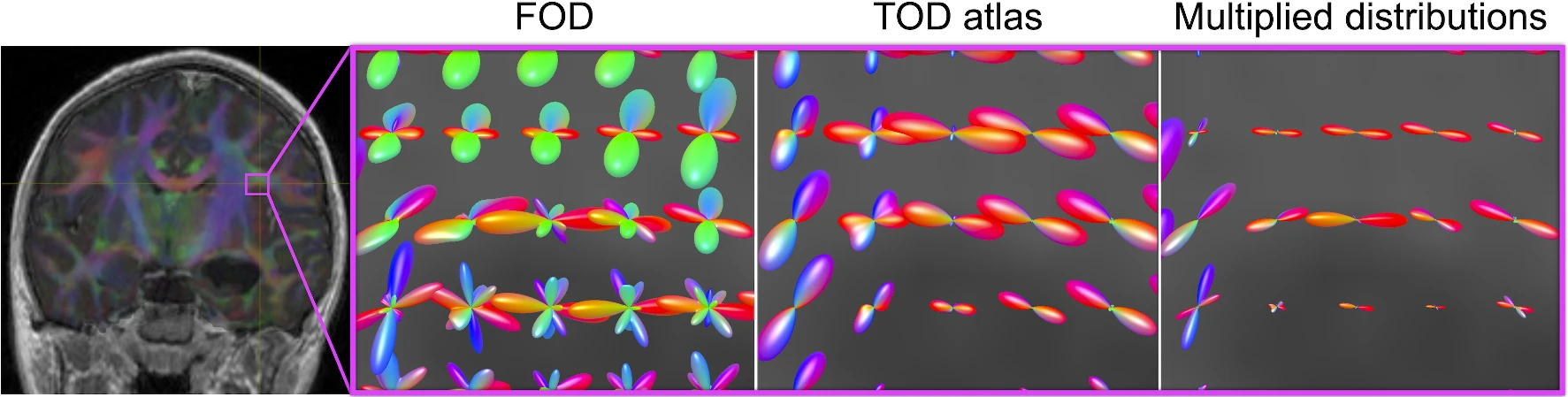
Unsupervised segmentation of the forceps major in four healthy adults across repeated scans (Young et al., Int J Comp Assist Radiol Surg, 2022).
Development, ageing and the connected brain
Brain development is a very complex and long drawn-out process, which continues well beyond birth. In particular, the formation of myelin around bundles of neuronal axons is reported to be a process which continues into adolescence, and possibly right up to the onset of adulthood. (This is an important aspect of brain development because the myelin sheath enables signals to be sent rapidly and reliably through the longer connective pathways.) It is by now well established that changes in the characteristics of white matter which are visible to diffusion MRI accompany normal postnatal development, indicating that white matter tracts gradually gain structural “coherence” over the years. Roughly opposite changes occur during one’s old age.
A few research groups have reported that many tracts tend to develop in tandem with one another. This may not seem surprising, since the brain grows in size during childhood and development of different parts of it may be expected to proceed in parallel, but the ability to use medical imaging to “observe” changes to white matter microstructure is very valuable. In our work, we have been able to separate out groups of tracts which “vary together”, and look at their relationships with age, gender and intelligence. We found that, over the age range of 8 to 16 years, age is the primary driver of change to white matter “integrity”, as expected, but the trajectory of change is substantially different between the two sexes. Even more interestingly, we observed a link between more subtle components of the diffusion MRI–based tract characteristics that we measured, and intelligence. These latter components were secondary to the age effect, and independent of age and gender. We have also shown a similar relationship between integrity in a group of tracts and cognitive processing speed in old age, as well as demonstrating that childhood intelligence has a substantial bearing on intelligence and the state of white matter in old age. These consistent links between particular white matter tracts and intelligence in early and late life paint a fascinating picture of the role of neural communication in facilitating cognition.
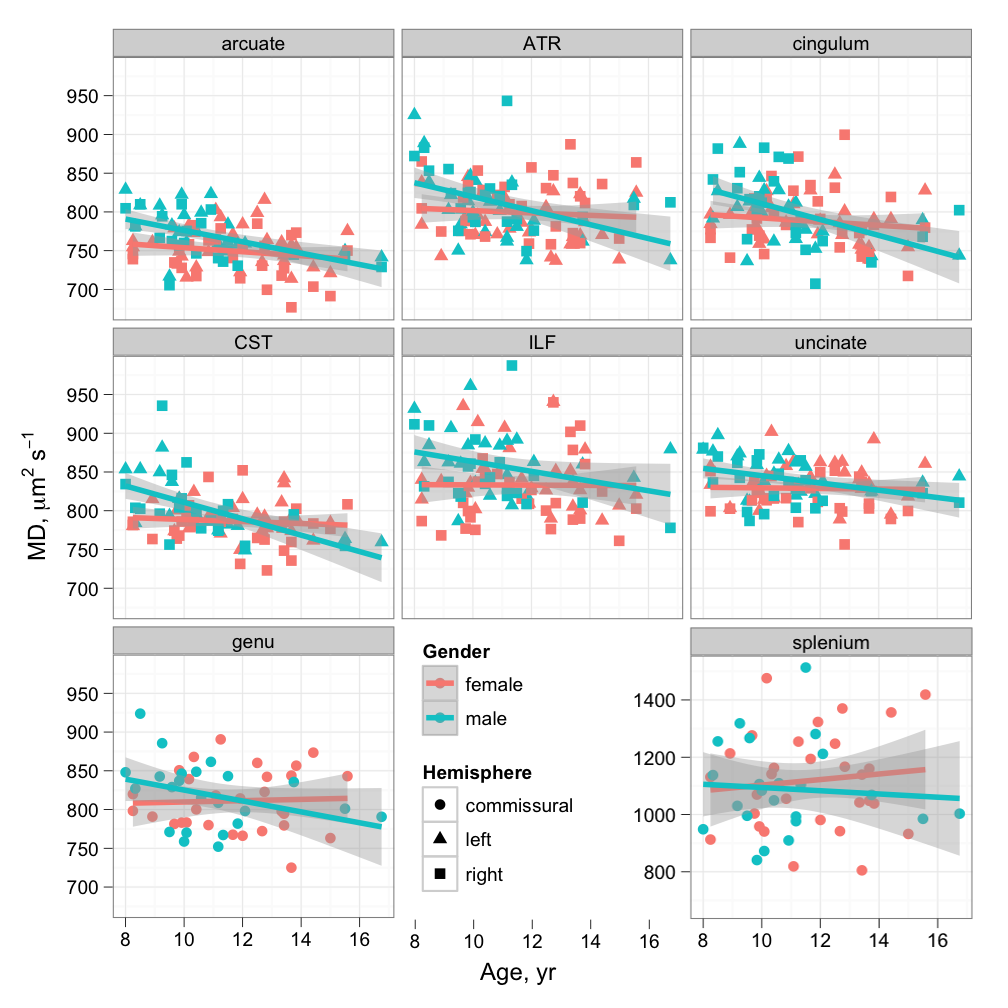
Cross-sectional age relationships of mean diffusivity in late childhood (Clayden et al., Cereb Cortex, 2012).
Multimodal brain networks
The interconnections between regions of the brain are vital to its function, and several neurological diseases are thought to be at least partly attributable to breakdowns in these communication channels. Medical imaging provides multiple windows into this network of connections, often called the “connectome”, and it has recently become common to represent these in terms of an abstract “graph”. I have reviewed the processes and pitfalls involved in using so-called structural data to reconstruct these connectomes, and we have found evidence of structural networks mediating the cognitive impact of sickle cell anaemia, but networks can also be built up using functional images, by looking for pairs of regions which are consistently active at similar times.
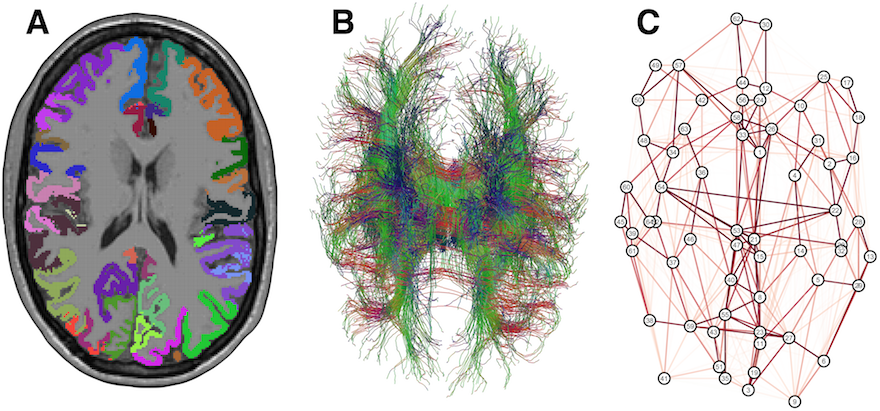
A cortical parcellation, combined with diffusion tractography, can be used to build a structural “connectome”, an abstract representation of interconnections in the brain (Clayden, Funct Neurol, 2013).
The structural and functional connectomes should be complementary, in the sense that they are two (admittedly limited) views of the same system. There is therefore a strong drive towards understanding how to combine the information most effectively. For our part, we have investigated the nature of the relationship between functional connectomes derived from different modalities, and between structural and functional connectomes, by attempting to predict one from the other consistently.
Although graphs in general are well understood—social networks, such as Facebook connections, are described using the same structures—there are reasons for treating brain networks specially. One such reason is that cognitive tasks do not necessarily use the whole brain, relying instead on functionally specialised subnetworks. One of our more technical developments was therefore to explore a novel way to subdivide brain networks, producing so-called “principal networks”. This technique is available in TractoR, and was quickly put to use in studies of children and adults with multiple sclerosis.
Bilingualism and the developing brain
There is evidence from many areas that growing up while systematically being exposed to more than one language has an effect on cognition that is broader than just language function. Through a detailed study of a group of children exposed to English and Greek, including cognitive and language testing alongside neuroimaging, we set out to investigate whether any differences between developing bilingual and monolingual brains might be due to varying patterns of maturation and/or variable language exposure. Some initial findings were featured in a BBC Ideas short film, and the full results are currently undergoing peer-review.
Alongside that work, we used the extensive dataset gathered over many years by the Millennium Cohort Study to investigate wider evidence of the effects of multiple language exposure. While this cohort study was not specifically set up with linguistic analysis in mind, and has important limitations, we still found indications that language exposure was relevant to broader cognitive domains in this much larger group, and importantly that the effect may depend on socioeconomic status.
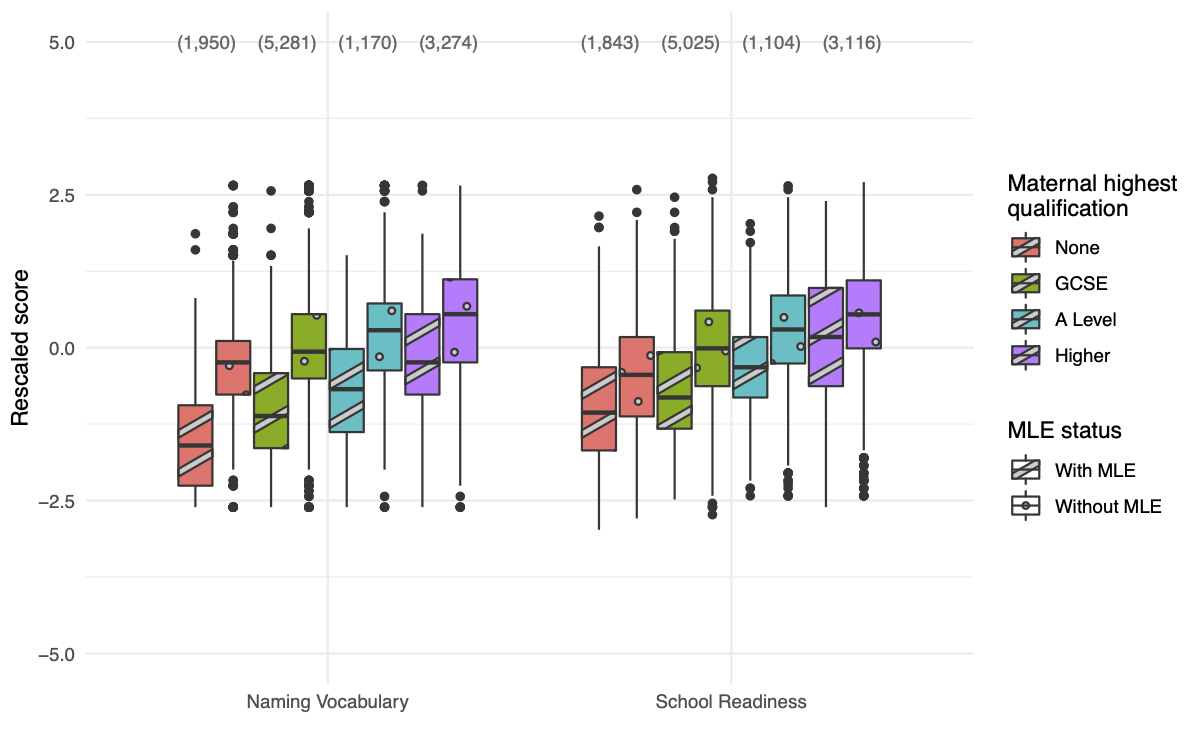
Boxplots of cognitive test scores gathered at age three from thousands of children, by maternal education level and whether the child was exposed to multiple languages (MLE) or not (Clayden et al., Front Psychol, 2023).
Copyright © 2025 Jon Clayden. Any views or opinions expressed on this site are my own and not those of UCL.