Mouse embryogenesis and genetics (Dr Hazel Smith)
The mouse
is not an immediately obvious choice of model system for developmental
biology. Its embryos are small, develop relatively slowly and are
inaccessible to experimental manipulation because most of development must take
place in utero. However, mice are at least as
easy to study as any other eutherian (placental - not
marsupial) mammal and, being mammals ourselves, we have a particular interest
in understanding mammalian development. The early stages of mammalian
development are quite different from those of other vertebrates such as Xenopus and so need to be studied in a mammal.
Moreover, mouse genetics is comparatively well developed (lots of mutants) and
techniques exist for manipulating gene function (knocking genes out and turning
them on), which are not available in any other model vertebrate.
Early
mouse development
The fertilized egg divides and develops as it travels along the oviduct to the uterus. In mice this process takes 4-5 days.The egg has a protective membrane, the zona pellucida, which stops it from implanting in the oviduct wall. By the time it reaches the uterus the egg has undergone many cell divisions to form a blastocyst, which hatches from the zona to implant into the uterine wall. Pre-implantation mouse development can be studied in culture - prior to implantation embryos can survive and can develop in liquid media.
One reason mammalian early development is
different from that of other vertebrates is that the extra-embryonic membranes
(amnion and chorion) play a much more
important role (they generate much of the placenta). Much of early
development involves deciding which cells will give rise to these
extra-embryonic membranes and which give rise to the embryo proper. Just before
it implants, the blastocyst is essentially an
asymmetric hollow ball of cells surrounding a fluid filled cavity - the blastocoel. Cells in the outer layer and the
inner mass have different fates. The outer layer of cells is called the trophectoderm and will give rise to the chorion. The inner cell mass or epiblast gives rise to the embryo proper. Inner cell
mass cells in contact with the blastocoel have a
distinct identity. They form the primitive endoderm and will give
rise to the amnion.
The process
by which cells become committed to become part of the embryo rather than of the
extra-embryonic membranes have been well characterized. Up to the eight
cell stage of development all cells are developmentally equal and totipotent (able to give rise to any type of cell,
embryonic or extra-embryonic). The evidence for this comes from two types
of experiment:-
1) From experiments isolating cells
from two-, four- or eight-cell embryos to see if each can give rise to a
complete individual (this can happen spontaneously to produce identical twins,
quads and, in sheep, octuplets).
2) From experiments where two
eight-cell embryos are fused and give rise two normal mice. The mice are chimeras,
meaning that they are composed of cells derived from two genetically distinct
individuals. If the cells had already committed to a particular fate you might
expect to produce a double embryo/placenta.
By the blastocyst stage, although they have lost the capacity to
form extra-embryonic membranes, any ICM cell can contribute to any embryonic
tissue - they are still totipotent with respect to embryonic
development (shown by generating chimeras by injecting ICM cells from one
strain of mice into a blastocycst from another).
Derivation of
embryonic stem (ES) cells
Targeted mutations in mice are performed by manipulating the genome of cultured cells derived from the ICM - Embryonic stem (ES) cells.
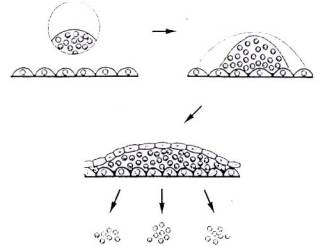
Cultured cells can be derived
by the following process:-
1)
Dissociation and plating. Most tissues, if
dissociated into single cells, can be plated out on specially treated surfaces
to which the cells become attached.
2) Attachment growth and
differentiation. When allowed to grow under liquid medium. Some of the
cells differentiate and lose the capacity to divide, others continue to divide.
3) Dissociation and replating. Dividing cells can be selected and
re-plated. This time a larger proportion of the cells will retain the
ability to divide.
4) Repeating this process eventually selects for cells which are able to divide indefinitely, unless exposed to media containing factors which force them to differentiate. These cells can be used to establish a cultured cell line. Most cell lines can differentiate to produce the cell type from which they were originally derived muscle to muscle cells, nerve to nerve cells. Cultured ES cells derived from the ICM can give rise to any cell type. Moreover, like ICM cells ES cells injected into blastocyst embryos can contribute to all tissues of the resulting chimeric adult including the germ cells -sperm or eggs.
Targeted mutagenesis
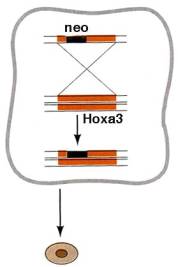
ES cells can be induced to take up foreign DNA
e.g. by electroporation. If this DNA includes
sequences with homology to endogenous mouse sequences it can be incorporated
into the ES cell genome by homologous recombination replacing the endogenous
sequence. This can be used to generate mice in which the coding sequence of a particular gene (e.g. Hoxa3) have been
replaced by sequences coding for a selectable marker such as the gene for
neomycin resistance. Cells in which this has happened (usually to only
one copy of the gene) will be the only ones to survive adding neomycin to the
culture medium. These cells, which are heterozygous mutant for the
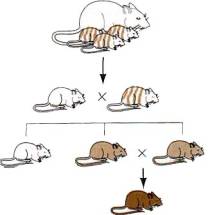
Hoxa3/neomycin deletion/replacement can be injected into a wild type blastocyst and re-implanted in a foster mother. The
mice derived from embryos that have undergone this procedure will be chimeric, composed of a mixture of mutant ES cell and wild
type host cells. However, if the ES cells have contributed to the germ line and given rise to mutant sperm or eggs, F1
offspring of the chimeras can be fully heterozygous mutant. Crossing
these F1 heterozygotes to each other can produce
homozygous Hoxa3 mutant mice whose phenotype can be analyzed to determine the
effects of developing in the absence of Hoxa3 protein.
Transgenesis
Transgenic
mice can be generated by simple microinjection of DNA into the nuclei of
fertilized eggs. Injected eggs are re-implanted in a foster mother (similar to
IVF in humans) and usually about 10% of the surviving offspring will carry an
insertion of the injected transgene in their
chromosomal DNA. Transgenes insert at random
locations in the genome (non-homologous recombination).
Transgenesis is used in research :-
*
to assess the function of tissue specific regulatory
sequences
*
to analyze the effect of misexpression
or overexpression
*
for insertional
mutagenesis
Insertional
mutagenesis occurs when the transgene inserts into an
endogenous mouse gene and disrupts its function. Even if the insertion has no
dominant phenotypic effect, heterozygote carriers can be identified by testing
for the presence of the transgene.
References
Wolpert
(1st edition) pp 37-41 and pp71-73
Scott Gilbert
(6th edition) pp 354-364
Molecular
Biology of the Cell (3rd edition) pp 1056-1059
The new
mouse genetics: altering the genome by gene targetting
M.R. Capecchi (1989) Trends in Genetics vol
5 pp70-76