back to publications
Oligodendrocyte development
William D Richardson
Wolfson Institute for Biomedical
Research and Department of Biology,
University College London, Gower
Street, London WC1E 6BT, UK
[in: Glial Cell Development. KR Jessen and WD
Richardson, editors. Oxford University Press. In press, 2000)
Correspondence: Bill Richardson / tel
+44 (0)20 7679 6729 / fax +44 (0)20 7209 0470 / w.richardson@ucl.ac.uk
Introduction
I
Specification of the oligodendrocyte lineage
Spinal
oligodendrocytes are derived from ventral neuroepithelium
Fig.1 Appearance and spread of PDGFRa +
oligodendrocyte progenitors in the embryonic mouse spinal cord
and forebrain
Fig. 2 Oligodendrocyte lineage markers in the E13.5 mouse
spinal cord
Spinal oligodendrocytes originate in the same part of
the neuroepithelium as motor neurons
Fig. 3 Regionalization of the ventral spinal cord VZ
Fig. 4 Possible lineages connecting motor neurons, O-2A
progenitors and astrocytes in the ventral spinal cord
Role of
Sonic hedgehog in oligodendrogenesis
How
does Shh work?
Negative
influences on oligodendrocyte lineage specification
Oligodendrogenesis in the
brain: analogies between forebrain and spinal cord
One or more
oligodendrocyte lineages in the forebrain?
Where do astrocytes
come from? Glial-restricted precursors and pre-progenitors
II
Control of cell proliferation and survival in the oligodendrocyte
lineage
Platelet-derived
growth factor (PDGF)
Fibroblast
growth factor (FGF)
Neurotrophins
Insulin and
insulin-like growth factors
Neuregulin/GGF
Chemokine
GRO-alpha
Other factors
III
Controls on oligodendrocyte differentiation
A
cell division limiter in oligodendrocyte progenitors: the role of
thyroid hormones
Cell-intrinsic
and cell-extrinsic controls on progenitor cell proliferation and
population growth
Conclusion
and outstanding questions
Fig. 5 Summary diagram of oligodendrocyte lineage
progression.
Reference
list
___________________________________________________________________________________________
Introduction
Oligodendrocytes, like other neural cells in
the central nervous system (CNS), develop from the
neuroepithelial precursors that line the lumen of the spinal cord
and the ventricles of the brain – the so-called ventricular
zone (VZ). Although myelinating oligodendrocytes are one of the
last cell types to differentiate in the CNS – accumulating
mainly after birth in rodents - their progenitors are present in
the VZ much earlier than that. In the rat spinal cord, for
example, the first overt oligodendrocyte precursors appear in the
VZ around embryonic day 14 (E14) or even earlier (conception is
E0, birth ~E21). Soon after this, they undergo a morphological
transition, detach from the epithelium and migrate way from the
VZ through the developing CNS (Reynolds and Wilkin, 1988; Levine
and Goldman, 1988). These migratory cells are referred to as O-2A
progenitor cells, to acknowledge the fact that they can
differentiate into either oligodendrocytes or "type-2"
astrocytes in vitro, depending on the composition of the culture
medium (Raff et al., 1983). O-2A progenitors were first
identified in rat optic nerve cell cultures (Raff et al., 1983);
similar (though not necessarily identical) cells are found in
cultures derived from many other parts of the CNS. O-2A glial
progenitors continue to divide after they leave the VZ, unlike
neuronal progenitors. Before they exit the cell cycle they
undergo a transition to pro-oligodendrocytes. This is
characterized by a change in morphology from a simple, often
bipolar form to a more complex form with multiple processes,
accompanied by a reduction in motility, a change in cell surface
antigens (notably acquisition of the O4 antigen in rats) and
altered growth factor responsiveness (Bansal and Pfeiffer, 1992).
The pro-oligodendrocytes are thought to undergo a late burst of
cell divisions near their site of terminal differentiation in
fibre tracts (Pfeiffer et al., 1994). They then leave the cell
cycle, associate with axons, express myelin gene products and
mature into fully-fledged oligodendrocytes. There are controls at
all stages of lineage development including specification,
proliferation, differentiation and long-term survival.
back to index
I Specification of the oligodendrocyte
lineage
Spinal oligodendrocytes are derived from
ventral neuroepithelium
Oligodendrocytes are distributed widely through
the adult mammalian central nervous system (CNS) with no obvious
preference for either dorsal-ventral or anterior-posterior
position. It has therefore been something of a surprise to
discover that the precursors of oligodendrocytes arise in highly
restricted parts of the VZ of the brain and spinal cord. It is
now clear that oligodendrocyte progenitors in the spinal cord and
brainstem are generated in a sub-domain of the ventral VZ near
the floor plate (Yu et al., 1994; Ono et al., 1995; Timsit et
al., 1995). Oligodendrocytes in the optic nerve develop from
progenitors that originate in a specialized part of the VZ in the
ventral diencephalon and migrate from there into the nerve via
the optic chiasm (Ono et al., 1997). Therefore, in these parts of
the neural tube at least, oligodendrocytes can be classified as a
ventral cell type.
The evidence for a ventral origin of
oligodendrocytes in the spinal cord has been reviewed before
(Miller, 1996; Hardy, 1997; Rogister et al., 1999; Richardson et
al., 2000; Thomas et al., 2000). There are three main types of
evidence:
1) Dorsal versus ventral spinal cord
cultures. When embryonic day 14 (E14) rat spinal cords are
bisected longitudinally into dorsal and ventral halves and the
cells from each half are cultured separately, oligodendrocytes
develop only in the ventral cultures (Warf et al., 1991). The
same is true for cultures of E6 chick or quail spinal cord
(Pringle et al., 1996; Orentas and Miller, 1996; Poncet et al.,
1996; Pringle et al., 1998). At later ages (E16 rat or E8 chick),
both dorsal and ventral cultures generate oligodendrocytes. This
is consistent with the idea that oligodendrocyte progenitors
first arise in the ventral half of the cord but later proliferate
and spread into the dorsal half.
2) Lineage markers in the spinal cord.
Several early markers of the oligodendrocyte lineage are
expressed in a restricted region of the ventral VZ at early
embryonic times (e.g. E14 rat, E12.5 mouse, E6 chick) and later
spread throughout the cord in a manner suggesting migration of
individual progenitor cells (see Fig. 1). The time course of
______________________________________________________________________________
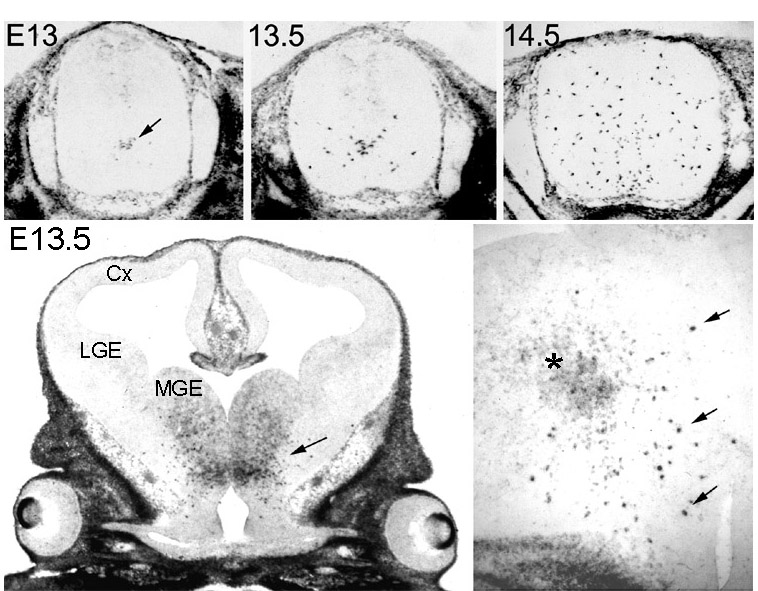 Figure 1 Appearance and spread of PDGFRa +
oligodendrocyte progenitors in the embryonic mouse spinal cord
and forebrain, visualized by in situ hybridization. In the spinal
cord the first progenitors appear in the ventral VZ on E13
(arrow), then they proliferate and migrate away from the midline.
Within a day-and-a-half (E14.5) they spread through most of the
cord. The equivalent ages in rat are E14 to E15.5 and in chick,
E7 to E9. In the forebrain (lower left), a cluster of PDGFRa +cells
appears in the ventral diencephalon (anterior hypothalamic
neuroepithelium) before E13 (arrow). These cells subsequently
proliferate and migrate into the dorsal forebrain including the
developing cortex (Cx) before birth (not shown). At higher
magnification (lower right) it can be seen that the migratory
cells are intensely labelled PDGFRa +
cells (arrows) that develop within the initial cluster of less
strongly labelled neuroepithelial cells (asterisk). MGE, medial
ganglionic eminence; LGE, lateral ganglionic eminence. back to index
Figure 1 Appearance and spread of PDGFRa +
oligodendrocyte progenitors in the embryonic mouse spinal cord
and forebrain, visualized by in situ hybridization. In the spinal
cord the first progenitors appear in the ventral VZ on E13
(arrow), then they proliferate and migrate away from the midline.
Within a day-and-a-half (E14.5) they spread through most of the
cord. The equivalent ages in rat are E14 to E15.5 and in chick,
E7 to E9. In the forebrain (lower left), a cluster of PDGFRa +cells
appears in the ventral diencephalon (anterior hypothalamic
neuroepithelium) before E13 (arrow). These cells subsequently
proliferate and migrate into the dorsal forebrain including the
developing cortex (Cx) before birth (not shown). At higher
magnification (lower right) it can be seen that the migratory
cells are intensely labelled PDGFRa +
cells (arrows) that develop within the initial cluster of less
strongly labelled neuroepithelial cells (asterisk). MGE, medial
ganglionic eminence; LGE, lateral ganglionic eminence. back to index
_____________________________________________________________________________________________
initial appearance and subsequent spread into
dorsal regions matches that predicted from the culture
experiments quoted above. Informative lineage markers include the
platelet-derived growth factor receptor-alpha (PDGFRa) (Pringle and
Richardson, 1993; Nishiyama et al., 1996), antigens recognized by
monoclonal antibody O4 (Ono et al., 1995), the NG-2 proteoglycan
(Stallcup and Beasley, 1987; Levine and Stallcup, 1987; Nishiyama
et al., 1996), transcripts encoding the myelin proteins cyclic
nucleotide phosphodiesterase (CNP) (Yu et al., 1994) and myelin
proteolipid protein (PLP/DM-20) (Timsit et al., 1995) and the
transcription factors Sox10, Olig-1 and Olig-2 (Kuhlbrodt et al.,
1998; Lu et al., 2000; Zhou et al., 2000) (more about these
later). Lineage markers have obvious shortcomings – few
individual markers are truly lineage-specific – but the
overlap among these markers is persuasive (e.g. Fig. 2).
PDGFRa+
cells in the late embryonic rat spinal cord are known to be O-2A
progenitors because if these cells are immunoselected with an
anti-PDGFRa antibody and cultured under appropriate conditions,
they all differentiate into oligodendrocytes or type-2 astrocytes
(Hall et al., 1996). Conversely, if PDGFRa+ cells are
selectively removed from cultures of rat spinal cord cells by
antibody-mediated complement lysis, then oligodendrocyte
production is strongly inhibited Hall et al., 1996). The same
goes for O4+ cells from embryonic chick spinal cord
(Ono et al., 1995)
_______________________________________________________________________________
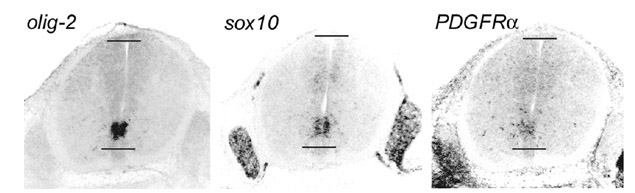 Figure 2 Oligodendrocyte lineage
markers in the E13.5 mouse spinal cord. Olig—2, sox10
and PDGFRa transcripts co-localize in the
ventral neuroepithelium. Note that olig—2 is
restricted to the CNS, sox10 is in both CNS and PNS (in
Schwann cells), while PDGFRa is
in mesodermal and ectodermal derivatives as well as the CNS.
Horizontal lines mark the ventral and dorsal limits of the
central canal. back to
index
Figure 2 Oligodendrocyte lineage
markers in the E13.5 mouse spinal cord. Olig—2, sox10
and PDGFRa transcripts co-localize in the
ventral neuroepithelium. Note that olig—2 is
restricted to the CNS, sox10 is in both CNS and PNS (in
Schwann cells), while PDGFRa is
in mesodermal and ectodermal derivatives as well as the CNS.
Horizontal lines mark the ventral and dorsal limits of the
central canal. back to
index
________________________________________________________________________________
3) Chick-quail chimeras. When dorsal or
ventral spinal cord neuroepithelium from a quail donor embryo is
grafted into the equivalent position of a chick host at the same
stage of development (homotypic, homochronic grafts), then
donor-derived oligodendrocytes develop only from ventral grafts
(Pringle et al., 1998). This is despite an earlier, misleading
report to the contrary (Cameron-Curry and Le Douarin, 1995).
Taken together, the evidence suggests strongly
that most or all oligodendrocytes in the spinal cord develop from
progenitor cells that originate from the ventral neuroepithelium.
back to index
Spinal oligodendrocytes originate in the
same part of the neuroepithelium as motor neurons
The localized origin of oligodendrocyte
progenitors in the ventral spinal cord naturally raises the
question of what makes the neuroepithelial precursors at that
site differ from their dorsal and ventral neighbours.
Fortunately, quite a lot is known already about how ventral
neurons are specified and some of this information is directly
applicable to oligodendrocytes.
Specification of ventral neurons – motor
neurons and several classes of ventral interneurons – is
known to depend on signal(s) from the notochord and floor plate
at the ventral midline (Tanabe and Jessell, 1996). An important
component of the signal is provided by Sonic hedgehog (Shh)
protein, a vertebrate homologue of the product of the Drosophila
segment-polarity gene hedgehog (hh). Shh from the
notochord first induces formation of the floor plate in the
adjacent neural tube, then the floor plate becomes a secondary
source of Shh which acts as a graded morphogen, diffusing
dorsally to establish a ventral-to-dorsal (high-low)
concentration gradient in the ventral neural tube. Shh, acting
through its cell-surface receptors Patched (Ptc) and Smoothened
(Smo), represses expression of the homeodomain transcription
factor Pax6, thus excluding Pax6 from the neuroepithelial domain
immediately abutting the floor plate and establishing a
ventral-to-dorsal (low-high) gradient of Pax6 in the remainder of
the ventral neuroepithelium (Ericson et al., 1997) (Fig. 3).
Pax6 regulates downstream genes, setting up discrete domains of
gene expression within the neuroepithelium. For example, Pax6
represses Nkx2.2 expression, which is therefore restricted to the
Pax6-negative domain abutting the floor plate (Ericson et al.,
1997). Other transcription factors that are presumably regulated
by Pax6 (and each other) include homeodomain proteins Nkx6.1 and
Dbx1 (Briscoe and Ericson, 1999), the high-mobility-group (HMG)
protein Sox10 (Kuhlbrodt et al., 1998) and the recently described
basic helix-loop-helix (bHLH) proteins Olig-1 and Olig-2 (Lu et
al., 2000; Zhou et al., 2000). Downstream of these is an
additional set of transcription factors, including the
homeodomain proteins Isl-1, Isl-2, Lim-3 Chx-10 and MNR2, which
are closely involved in the differentiation of post-mitotic
neurons after they have left the VZ (Briscoe and Ericson, 1999).
Neurogenesis therefore depends on a hierarchy of protein-gene
interactions initiated by a morphogenic gradient of [Shh]. This
is analogous to the way the segmented structure of the Drosophila
embryo becomes established along its anterior-posterior axis
(though in this example the primary morphogen is Bicoid protein,
not Hedgehog).
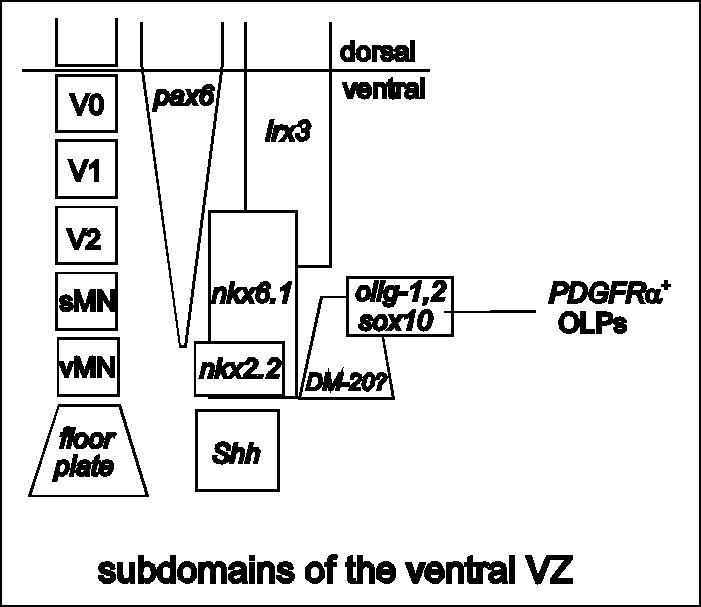 Figure 3
Regionalization of the ventral spinal cord VZ. On the left,
separate blocks of neuroepithelium give rise to different cell
types. The block nearest the floor plate generates visceral motor
neurons (vMNs), then successively more dorsal blocks give rise to
somatic motor neurons (sMNs) and ventral interneurons V2, V1 and
V0. These neuroepithelial domains are marked by their distinctive
patterns of gene expression. The vMN domain is defined by nkx2.2
while more dorsal regions express one or more of nkx6.1, pax6,
irx3 to name a few (Briscoe and Ericson, 1999). This
pattern is established before the onset of neurogenesis about
E10. Graded Shh is thought to induce an inverse gradient of Pax6
which in turn controls downstream genes in a
concentration-dependent manner. Of particular note, the olig—1,
olig—2, sox10 domain corresponds to that part of the VZ
that gives rise to somatic MNs. Later (~E13) this domain gives
rise to migratory PDGFRa + oligodendrocyte progenitors
(OLPs). PLP/DM—20 is expressed weakly in the ventral VZ from
before E11 (W.-P. Yu and WDR, unpublished); the ventral limit of
expression is close to the floor plate but the dorsal limit is
not well defined. back
to index
Figure 3
Regionalization of the ventral spinal cord VZ. On the left,
separate blocks of neuroepithelium give rise to different cell
types. The block nearest the floor plate generates visceral motor
neurons (vMNs), then successively more dorsal blocks give rise to
somatic motor neurons (sMNs) and ventral interneurons V2, V1 and
V0. These neuroepithelial domains are marked by their distinctive
patterns of gene expression. The vMN domain is defined by nkx2.2
while more dorsal regions express one or more of nkx6.1, pax6,
irx3 to name a few (Briscoe and Ericson, 1999). This
pattern is established before the onset of neurogenesis about
E10. Graded Shh is thought to induce an inverse gradient of Pax6
which in turn controls downstream genes in a
concentration-dependent manner. Of particular note, the olig—1,
olig—2, sox10 domain corresponds to that part of the VZ
that gives rise to somatic MNs. Later (~E13) this domain gives
rise to migratory PDGFRa + oligodendrocyte progenitors
(OLPs). PLP/DM—20 is expressed weakly in the ventral VZ from
before E11 (W.-P. Yu and WDR, unpublished); the ventral limit of
expression is close to the floor plate but the dorsal limit is
not well defined. back
to index
__________________________________________________________________________
Therefore, under the action of Shh the
neuroepithelium is subdivided along the dorsal-ventral axis, each
division generating a different subset of cell types
(Fig. 3). The domain nearest the floor plate (Pax6-negative,
Nkx2.2-positive,) generates visceral (autonomic) motor neurons,
which innervate muscles of the heart or diaphragm, for example.
The next more dorsal domain (Pax6-low, Nkx2.2-negative) generates
somatic motor neurons. In the brainstem these innervate
craniofacial muscles such as those of the tongue (hypoglossal
motor neurons), while in the cervical spinal cord they innervate
skeletal (axial) muscles in the neck. Further dorsal again
(Pax6-positive, Nkx2.2.-negative), consecutive domains of
neuroepithelium generate V2, V1 and V0 interneurons. This takes
us to the dorsal-ventral midline, where the organizing influence
of the floor plate cedes to that of the roof plate (Ericson et
al., 1997; Briscoe and Ericson, 1999).
In the rodent, PDGFRa+
oligodendrocyte progenitors appear to arise in the same Pax6-low,
Nkx2.2-negative region of the neuroepithelium that generates
somatic motor neurons (Sun et al., 1998; Lu et al., 2000),
implying some kind of lineage relationship between motor neurons
and oligodendrocytes. The idea of a common motor
neuron-oligodendrocyte lineage is consistent with clonal analysis
in the embryonic chick spinal cord in vivo (Leber et al., 1990;
Leber and Sanes, 1995), and in the rat spinal cord in vitro
(Kalyani et al., 1997). The precise form of the lineage tree that
connects these cell types is unknown, but any lineage model must
take into account the fact that oligodendrocyte progenitors are
generated after motor neurons. In the rat, for example,
post-mitotic motor neurons are born between E11 and E13 (Nornes
and Das, 1974) whereas PDGFRa+
progenitors do not appear until E14 (Pringle and Richardson,
1993). One possibility is that motor neuron precursors and PDGFRa-negative
oligodendrocyte "pre-progenitors" are segregated early
on in the VZ through division of neuroglial precursors, and the
oligodendrocyte pre-progenitors then remain dormant in the VZ
until after motor neuron production is complete. Alternatively,
motor neuron and oligodendrocyte progenitors might be formed
sequentially from a common neuroglioblast in the VZ (Richardson
et al., 2000) (see Fig. 4).
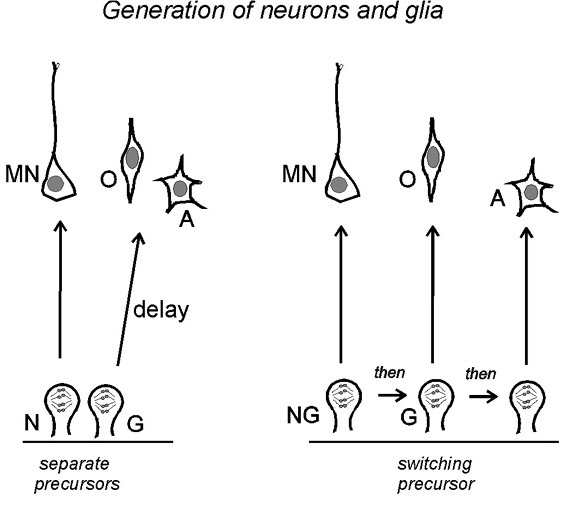 Figure 4 Two possible cell lineages connecting
motor neurons (MN), O-2A progenitors (O) and astrocytes (A) in
the ventral spinal cord. Left: separate neuroblasts (N)
and glioblasts (G) exist side-by-side in the neuroepithelium from
early times; the glioblast lies dormant during motor neuron
production and is activated later. The separate N and G cells
would be formed by asymmetric division of an earlier NG
neuroepithelial cell. Right: a single population of
neurogliobasts (NG) switches fates with time or successive
divisions to generate motor neurons, O-2A progenitors and
astrocytes sequentially. Other schemes combining elements of both
models are also possible. back
to index
Figure 4 Two possible cell lineages connecting
motor neurons (MN), O-2A progenitors (O) and astrocytes (A) in
the ventral spinal cord. Left: separate neuroblasts (N)
and glioblasts (G) exist side-by-side in the neuroepithelium from
early times; the glioblast lies dormant during motor neuron
production and is activated later. The separate N and G cells
would be formed by asymmetric division of an earlier NG
neuroepithelial cell. Right: a single population of
neurogliobasts (NG) switches fates with time or successive
divisions to generate motor neurons, O-2A progenitors and
astrocytes sequentially. Other schemes combining elements of both
models are also possible. back
to index
________________________________________________________________________________________________________
Whatever the form of the relationship, one idea
worth exploring is that production of PDGFRa+
oligodendrocyte progenitors might be triggered by feedback
signals from newly-generated motor neurons. This could explain
why oligodendrocyte progenitors appear after motor neurons.
However, mature motor neurons do not seem to be required for
oligodendrogenesis, because oligodendrocytes are generated as
normal in explant cultures of spinal cords from Isl-1
knockout mice, which fail to develop any motor neurons (Sun et
al., 1998). Nevertheless, it remains possible that motor neuron progenitors,
which do not express Isl-1 and presumably are not affected
in the Isl-1 knockouts, might provide a feedback signal.
Motor neuron progenitors lie closer to the VZ than differentiated
motor neurons and might therefore be better placed to signal back
to the neuroepithelium.
back to index
Role of Sonic hedgehog in oligodendrogenesis
Explant cultures of dorsal or intermediate
spinal cord neuroepithelium, which on their own do not give rise
to any ventral cell types, can be induced by pure recombinant Shh
to give rise to a variety of ventral cells including floor plate
cells, motor neurons and oligodendrocytes (Roelink et al., 1994;
Pringle et al., 1996; Poncet et al., 1996; Ericson et al., 1997;
Orentas et al., 1999). The concentrations of Shh required to
induce motor neurons and oligodendrocytes are similar - and lower
than needed to induce floor plate cells - consistent with the
putative gradient of [Shh] in the ventral neural tube and also
with the coincident origin of somatic motor neurons and
oligodendrocytes in vivo (Pringle et al., 1996; Orentas et al.,
1999). Shh is necessary for oligodendrogenesis in vivo, because
oligodendrocytes do not develop in chick spinal cords that are
exposed to anti-Shh neutralizing antibody in ovo (Orentas et al.,
1999). Moreover, PDGFRa+ oligodendrocyte progenitors do not appear
in the spinal cords of perinatal Shh null mice (personal
communication from Christer Betsholtz, University of Göteborg,
Sweden).
Two periods of Shh exposure are required for
motor neuron induction - an early exposure to ventralize the
spinal cord and another closer to the time of motor neuron
production (Ericson et al., 1996). Oligodendrocyte specification
requires an extended period of exposure throughout the time of
motor neuron production until shortly before the appearance of
the first O4-positive progenitor cells in chick ventral spinal
cord explants (Orentas et al., 1999; N.P Pringle unpublished). It
is not clear what this implies for the mode of action of Shh.
How does Shh work? back to index
Constitutive activation of Shh signal
transduction pathways through mutation or deletion of its
inhibitory receptor Ptc results in tumour formation in mice and
humans, implying that Shh signalling positively regulates the
cell cycle (Stone et al., 1996; Goodrich et al., 1997). In vitro,
Shh can act as a mitogen for neural precursor cells from
cerebellum, retina and spinal cord (Levine et al., 1997; Jensen
and Wallace, 1997; Dahmane and Ruiz i Altaba, 1999; Rowitch et
al., 1999). However, the concentration of Shh required to induce
DNA synthesis in vitro is generally higher than is needed to
specify cell fates in vitro. It could be that the mitogenic
effect of Shh is spurious, resulting from cross-talk between
intracellular signal transduction pathways at excessively high,
non-physiological levels of Shh signalling. On the other hand,
neutralizing anti-Shh antibodies have been reported to reduce
bromodeoxyuridine (BrdU) incorporation in vivo, implying a
physiological role for Shh in stimulating DNA synthesis (Dahmane
and Ruiz i Altaba, 1999; Wallace and Raff, 1999).
A key intracellular transducer of Hedgehog
signalling in Drosophila is the transcription factor
Cubitus interruptus (Ci). The vertebrate equivalent is the Gli
family of proteins, three of which have been described to date
(Ruiz i Altaba, 1999). The Gli proteins mediate different aspects
of Shh signalling and have both complementary and antagonistic
activities (Matise et al., 1998; Ruiz i Altaba, 1998). All three
are expressed in the ventral neural tube. Ventral neurons appear
to develop normally in mice lacking Gli1, Gli2 or both, even
though Gli2-deficient mice lack a floor plate (Matise et al.,
1998). Mice with a Gli3 mutation (Gli3xt)
have defects in the dorsal neural tube but no obvious ventral
defects (Hui and Joyner, 1993). The Gli family is named for the
fact that Gli1 was first identified as the product of a
gene amplified in human glioma (Kinzler et al., 1987), suggesting
a link with glial cell biology. However, the possibility that
glial development might be specifically affected in Gli-deficient
animals has not yet been examined. Intriguingly, Gli1 is
expressed in the ventral neuroepithelium of the mouse spinal cord
close to the time and place where PDGFRa +
oligodendrocyte progenitors first arise (Ruiz i Altaba, 1998).
back to index
Negative influences on oligodendrocyte
lineage specification
Oligodendrocyte development is subject to
negative as well as positive control. For example, bone
morphogenetic proteins (BMPs) inhibit oligodendrocyte development
and promote astrocyte development in cultures of embryonic
precursor cells from cerebral cortex, while the BMP inhibitor
Noggin has the opposite effect (Zhu et al., 1999; Mabie et al.,
1999; Mehler et al., 2000). BMPs and Dorsalin, another member of
the extended transforming growth factor beta (TGFb ) family, are
expressed in the dorsal neural tube and/or adjacent epidermis and
are known to influence differentiation of dorsal cells (Basler et
al., 1993; Liem et al., 1995; Lee and Jessell, 1999). These or
related factors might normally be required to inhibit
oligodendrogenesis in the dorsal neural tube.
back to index
Oligodendrogenesis in the brain
Analogies between forebrain and spinal cord
Oligodendrogenesis at more anterior levels of
the neuraxis has not yet been studied as extensively as in the
spinal cord and a consensus has yet to be reached. However, what
we know suggests that there might be strong analogies between
oligodendrogenesis in the embryonic spinal cord and forebrain.
1. Dorsal versus ventral forebrain cultures.
Cell cultures established from E14 or E15 rat cerebral cortex
(i.e. dorsal forebrain) have a much lower capacity for generating
oligodendrocytes than do equivalent cultures of ventral forebrain
(Birling and Price, 1998; Tekki-Kessaris et al., 2000). Moreover,
fragments of E15 cortex transplanted into the eye do not produce
myelinating cells (Kalman and Tuba, 1998). In contrast, E17 or
E18 cortical cells generate oligodendrocytes readily in culture
and also give rise to ectopic myelin when transplanted into the
eye. In principle, these experiments resemble the earlier spinal
cord experiments of Warf et al. (1991) (see above) and can be
interpreted in a similar way – that oligodendrocyte
progenitors originate in the ventral forebrain and later migrate
dorsally into the cerebral cortex.
2. Lineage markers in the forebrain. The
myelin gene PLP/DM-20 is expressed in the neuroepithelium
of the ventral diencephalon from as early as E9 in the mouse
(Timsit et al., 1992). In addition, neuroepithelial cells in the
pre-optic area of the ventral diencephalon (beneath the medial
ganglionic eminence) express PDGFRa weakly from around
E12 in the mouse (E13 rat) (Tekki-Kessaris et al., 2000)
(Fig. 1). Subsequently, small, strongly PDGFRa-labelled
cells appear in the neuroepithelium, increasing in number and
spreading laterally and dorsally into all parts of the forebrain,
including the cerebral cortex, before birth (Tekki-Kessaris et
al., 2000). These PDGFRa+ cells have been purified from late
embryonic (E19) rat forebrain and cerebral cortex by
immunoselection with an anti-PDGFRa Ig (Tekki-Kessaris et al.,
2000). Like their counterparts in spinal cord and optic nerve,
they can all differentiate into oligodendrocytes in culture. At
E12 in the mouse, olig-1 and sox10 are co-expressed
in the ventral forebrain neuroepithelium with PDGFRa. Olig-2
is more widespread, though still restricted to the ventral
diencephalon (Tekki-Kessaris et al., 2000). Thus established
lineage markers indicate a ventral origin for oligodendrocytes in
the forebrain.
3. Chick-quail chimeras. Direct evidence
that dorsal forebrain precursors do not normally generate
oligodendrocytes comes from chick-quail chimeras. When a fragment
of quail dorsal prosencephalon is grafted into the equivalent
position of a chick embryo at the same stage of development, the
quail graft can integrate seamlessly into the host and
contributes to neurogenesis in the chimeric cortex. However, no
donor-derived (quail) oligodendrocytes develop in such chimeras
(personal communication from Salvador Martinez, University of
Murcia, Spain).
There is no floor plate per se in the
forebrain, but nevertheless Shh is expressed in the
neuroepithelium at the ventral midline of the diencephalon,
adjacent to the region of olig-1, sox10, PDGFRa
co-expression, suggesting that Shh is involved in oligodendrocyte
lineage specification in the forebrain as in the spinal cord. In
support of this, production of oligodendrocytes and their
progenitors in explant cultures of rat or chick prosencephalon is
inhibited by anti-Shh neutralizing antibodies (Tekki-Kessaris et
al., 2000). It is not useful to look in Shh null mice as
these animals lack a recognizable forebrain. However, in nkx2.1
null mice Shh expression in the ventral diencephalon is
specifically ablated, leaving more caudal sites of expression
intact (Sussel et al., 1999). These mice have a morphologically
normal forebrain. They die at birth but nevertheless allow us to
investigate early oligodendrogenesis in the forebrain in the
absence of Shh. We found that (PDGFRa+, Sox10+)
cells do not appear on cue (E12) in the ventral forebrains of nkx2.1
null mice, although they do appear later, possibly by migration
from more caudal regions (Tekki-Kessaris et al., 2000). Overall,
the evidence supports the view that oligodendrocytes in the
embryonic forebrain develop from migratory PDGFRa+
progenitors that are generated in the ventral neuroepithelium by
a Shh-dependent process, as in the spinal cord.
Naturally, oligodendrocytes in the forebrain
cannot be lineally related to motor neurons as they appear to be
in the spinal cord, since there are no motor neurons in the
forebrain. However, it remains possible that oligodendrocytes are
related to some analogous class of neurons in the forebrain,
perhaps evolutionarily related to motor neurons. There are
neurons in the ventral diencephalon that are involved in motor
control, for example.
back to index
One or more oligodendrocyte lineages in the
forebrain?
Neuroepithelial cells in the ventral
diencephalon express the myelin gene PLP/DM-20 from early
times (e.g. before E9 in the mouse) and it has been proposed that
even at these early times these cells might be dedicated
oligodendrocyte precursors (Timsit et al., 1992; Ikenaka et al.,
1993; Timsit et al., 1995; Spassky et al., 1998; Perez et al.,
1999; Spassky et al., 2000). The relationship between these PLP/DM-20+
neuroepithelial cells and the PDGFRa+
neuroepithelial cells that appear later in the diencephalon (E12
mouse, see above) is still unclear. It might turn out that they
are one and the same cell population, or one might be contained
within the other.
The picture is complicated because a different
population of PLP/DM-20 cells appears later during
embryonic development of the mouse (Timsit et al., 1995;
Dickinson et al., 1996; Fanarraga et al., 1996; Peyron et al.,
1997). In contrast to the weakly labelled, tight-packed
neuroepithelial cells discussed above, these are strongly
labelled small cells outside the VZ. Relatively small numbers of
these latter cells are present in the brainstem of the mouse from
E12.5 and in the spinal cord from E14. Similar cells are also
present in the ventral diencephalon of the chick (Perez et al.,
1999). These PLP/DM-20+ cells do not
co-express PDGFRa and on that basis, together with their early
appearance, it has been suggested that they might be progenitors
of a novel oligodendrocyte lineage (Spassky et al., 1998; Perez
et al., 1999; Spassky et al., 2000). However, they do not readily
incorporate bromodeoxyuridine (BrdU) as would be expected of
proliferating progenitors; nor do they increase in number
noticeably in the mouse spinal cord between E14 and E17 (Hardy
and Friedrich, 1996; Fruttiger et al., 1999). They co-express
mRNA encoding MBP and CNP (Peyron et al., 1997) and in this and
their complex, process-bearing morphology they resemble
differentiating oligodendrocytes. One possibility is that the PLP/DM-20+
cells are post-mitotic, non-myelinating (or pre-myelinating)
oligodendrocytes formed by precocious differentiation of PDGFRa+
progenitors. PDGFRa is known to be rapidly down-regulated during
oligodendrocyte differentiation (Hart et al., 1989; Hall et al.,
1996; Butt et al., 1997a), which would explain the lack of
overlap between PDGFRa and PLP/DM-20 expression.
back to index
Where do astrocytes come from?
Glial-restricted precursors and pre-progenitors
We have emphasized the developmental
connections between oligodendrocytes and neurons. Where do
astrocytes fit in? Glial-restricted precursors (GRPs) that can
generate both astrocytes and oligodendrocytes have been isolated
from E13.5 rat spinal cord by immunoselection with the A2B5
monoclonal antibody (Rao et al., 1998). In clonal density
cultures, each of these A2B5+ cells could give rise to
oligodendrocytes and one of two types of astrocytes (A2B5+
or A2B5—), depending on culture conditions. At
E13.5, A2B5 immunoreactivity was detected in a large part of the
central spinal cord neuroepithelium overlapping, but not
restricted to, the oligodendrogenic domain defined by the olig
genes and PDGFRa (Rao et al., 1998). This is consistent with the finding
of Hall et al. (1996) that many A2B5+ cells in E14 rat
spinal cord cultures do not co-express PDGFRa. Whether all the A2B5+
GRPs studied by Rao et al. (1998) generated oligodendrocytes via
a PDGFRa+
intermediate is not known.
Pre-progenitor cells that can give rise to
oligodendrocyte (O-2A-like) progenitors have also been described
in mixed cell cultures established from neonatal rat cerebral
hemispheres. These cells are responsive to PDGF and express the
polysialylated form of the neural cell adhesion molecule
(PSA-NCAM) (Grinspan et al., 1990; Ben-Hur et al., 1998; Vitry et
al., 1999). They can be purified from mixed neural cultures by
immunoselection with an antibody against PSA-NCAM and stimulated
to proliferate as free-floating clusters ("spheres")
with thyroid hormone (TH) together with fibroblast growth factor
(FGF) or PDGF. When induced to differentiate on an adherent
substratum, the PSA-NCAM pre-progenitors generate mainly
oligodendrocytes and astrocytes so in this respect they resemble
the GRPs discussed above (Ben-Hur et al., 1998). Apart from their
different tissue of origin (brain versus spinal cord), the
PSA-NCAM+ pre-progenitors differ from GRPs in that
they express PDGFRa immunoreactivity and survive and proliferate in
response to PDGF (Grinspan and Franceschini, 1995; Ben-Hur et
al., 1998), whereas GRPs do not (Rao et al., 1998).
Retroviral lineage studies frequently indicate
that astrocytes and oligodendrocytes are generated from separate
progenitors in vivo (Parnavelas et al., 1991; Grove et al., 1993;
Williams and Price, 1995). However, pluripotent precursors that
can generate oligodendrocytes, astrocytes and even neurons have
also been described (Leber et al., 1990; Galileo et al., 1990;
Parnavelas et al., 1991; Williams et al., 1991; Levison and
Goldman, 1997). Common oligodendrocyte-astrocyte precursors (some
of which also generate neurons) have been identified following
stereotactic injection of retroviral vectors into the
subventricular zones (SVZ) that underlie the lateral tips of the
lateral ventricles of the postnatal forebrain (Levison and
Goldman, 1993; Zerlin et al., 1995; Levison and Goldman, 1997)
(see Chapter X by Goldman). How these precursors in the postnatal
SVZ relate to the A2B5+ GRPs or PSA-NCAM+
pre-progenitors discussed above remains to be determined.
back to index
II Control of cell proliferation and
survival in the oligodendrocyte lineage
Since the first discovery of oligodendrocyte
(O-2A) progenitors in rat optic nerve cell cultures (Raff et al.,
1983), attention has focussed on the factors that control their
proliferation. This is because it was recognized early on that
proliferation and differentiation are usually mutually exclusive,
so that the key to understanding oligodendrocyte differentiation
lies in unravelling the control of progenitor cell proliferation
and cell cycle exit (Raff et al., 1985). Later, it became clear
that proliferation is only part of the story, and that
extracellular signals are required continuously in order to
prevent apoptotic death of oligodendrocyte lineage cells (Barres
et al., 1992; reviewed by Raff et al., 1993). In the following
section I review what we know about the control of proliferation
and survival, concentrating on the roles of extracellular
signalling molecules.
Platelet-derived growth factor (PDGF)
PDGF is mitogenic for O-2A progenitors in vitro
(Raff et al., 1988; Richardson et al., 1988; Noble et al., 1988)
and in vivo (Calver et al., 1998; Fruttiger et al., 1999).
Presently we recognize three isoforms of PDGF (PDGF-A, -B, -C),
and two receptors (PDGFRa, PDGFRb ). All three PDGF isoforms bind to PDGFRa, whereas only
PDGF-B binds PDGFRb at high affinity (Heldin and Westermark, 1989; Li et
al., 2000). Active PDGF is a covalent homo- or hetero-dimer.
PDGF-A-containing dimers acting through PDGFRa on progenitor
cells are responsible for most of the PDGF-mediated mitogenic
response in the spinal cord, optic nerve and cerebellum, since
knockout mice lacking the PDGF A-chain have profoundly reduced
numbers of progenitors in these tissues (Fruttiger et al., 1999).
PDGF-B seems much less important, because PDGF-B null mice
have normal numbers of progenitors (Fruttiger et al., 1999).
PDGF-C might play a role because there is a relatively modest
(~50%) reduction in progenitor cell numbers in the brainstem of
the PDGF-A null mouse, which might indicate residual
activity of PDGF-CC, or an unrelated factor such as
neuregulin/glial growth factor (NRG/GGF, see below).
PDGF-A is made by many neurons in the CNS and
also by astrocytes (Yeh et al., 1991; Mudhar et al., 1993;
Ellison et al., 1996). Transgenic mice expressing a PDGF-A
transgene in neurons under the control of the neuron-specific
enolase promoter (NSE-PDGF-A mice) have many more PDGFRa+
oligodendrocyte progenitors than normal in grey matter areas
(Calver et al., 1998), but normal numbers in the optic nerve, an
isolated white matter tract (Fruttiger et al., 2000). In
contrast, transgenic mice that express PDGF-A under the GFAP
promoter (GFAP-PDGF-A mice) have greatly increased
progenitor cell numbers everywhere including the optic nerve,
which is visibly hypertrophic as a result (Fruttiger et al.,
2000). This suggests that PDGF-AA can be delivered into the optic
nerve (and other white matter tracts) by astrocytes that reside
within the nerve, but not from the axons of neurons that project
through the nerve. This might apply to other diffusible factors
that are synthesized by neurons. For neurons to deliver factors
directly into axon tracts it might be that the factors need to be
surface-bound, and transferred into the nerve by lateral
diffusion or transport in the plane of the axonal membrane.
The fact that oligodendrocyte progenitor
numbers are increased in PDGF-A transgenic mice
demonstrates that PDGF is present in limiting concentrations in
the normal developing CNS. This conclusion is also supported by
the observation that exposure to saturating concentrations of
PDGF in vitro causes freshly immuno-purified O4+
pro-oligodendrocytes to revert transiently to O4—
progenitors (Gard and Pfeiffer, 1993). The fact that PDGF is
normally in short supply is likely to keep progenitor cell
division in check, contributing to cell cycle exit and
oligodendrocyte differentiation in vivo (see below).
Although white matter astrocytes synthesize
PDGF mRNA and protein, they might not secrete PDGF
constitutively. O-2A progenitors do not proliferate when optic
nerve cell cultures are maintained in defined medium including
high concentrations of insulin but without added PDGF, even
though optic nerve astrocytes in the cultures themselves make
PDGF. However, adding pure PDGF to the cultures induces O-2A
progenitor proliferation (e.g. Richardson et al., 1988; Robinson
and Miller, 1996). This suggests that optic nerve astrocytes do
not release PDGF constitutively in culture. Preventing action
potential propagation through the optic nerve in vivo by
injecting tetrodotoxin (TTX, a sodium channel blocker) into the
eye inhibits O-2A progenitor cell proliferation in the optic
nerve; this inhibition can be overcome by simultaneously
delivering exogenous PDGF-AA to the nerve (Barres and Raff,
1993). Together, these data raise the possibility that release of
PDGF and/or other factors from optic nerve astrocytes might be
regulated by electrical activity in retinal ganglion cell axons
(Barres and Raff, 1993).
Note that PDGF by itself is not a particularly
potent mitogen for purified O-2A progenitors (Barres and Raff,
1994; Robinson and Miller, 1996). Additional polypeptide growth
factors can potentiate the activity of PDGF; frequently this
effect is masked because other cells (e.g. astrocytes) that are
present in the cultures provide co-factors. Therefore, while PDGF
is undoubtedly crucial for cell proliferation in vivo, it
normally acts in combination with other factors (see below).
When O-2A progenitors start to differentiate
into oligodendrocytes, they rapidly lose PDGF receptors.
Immunoreactive PDGFRa cannot be detected on newly-formed oligodendrocytes, or
even on the majority of O4+ pro-oligodendrocytes in
culture (Hall et al., 1996). This explains why PDGF is not
mitogenic for pro-oligodendrocytes or oligodendrocytes in culture
(Gard and Pfeiffer, 1990; Gard and Pfeiffer, 1993). Nevertheless,
PDGF can still act as a survival factor for newly formed GC+
oligodendrocytes (Barres et al., 1992; Barres et al., 1993a).
Much lower levels of PDGF signalling are needed to stimulate
survival versus proliferation of O-2A progenitors in vitro
(Barres et al., 1993a) so very low, even undetectable amounts of
PDGFRa
on newly-formed oligodendrocytes might be adequate for a survival
response. Oligodendrocytes that are more than a few days old
cannot any longer be kept alive in vitro by PDGF (Barres et al.,
1992; Barres et al., 1993a), presumably because they eventually
lose their PDGF receptors altogether. They evidently do not
dismantle their mitotic machinery irreversibly because some
factors like neuregulin/glial growth factor (NRG/GGF) can induce
them to re-enter the division cycle (Canoll et al., 1996) (see
below).
Myelinating oligodendrocytes presumably depend
on factors other than PDGF for their survival in vivo. This is
strikingly illustrated by our NSE-PDGF-A transgenic mice.
Although these animals contain many more progenitor cells and
produce many more immature oligodendrocytes than normal, the
additional oligodendrocytes are all removed by programmed cell
death (PCD) soon after they are formed so that the final number
of mature, myelin-forming oligodendrocytes is the same as in wild
type animals (Calver et al., 1998). Similar data have been
obtained by injection of PDGF-AA into the cerebrospinal fluid of
new-born rats (Butt et al., 1997b); in those experiments,
oligodendrocyte differentiation and myelination in the anterior
medullary vellum (AMV) was found to be retarded, presumably due
to prolonged progenitor cell proliferation, but the ultimate
number of myelinating oligodendrocytes was unaltered. PDGF-AA is
clearly not a long-term survival factor for differentiated
oligodendrocytes.
It is likely that survival factors for
oligodendrocytes are provided by the axons that they ensheath, so
that oligodendrocyte number is matched to the surface area of
axons to be myelinated (Barres and Raff, 1994). Since there is no
evidence that either axon number or diameter is increased by
augmenting the supply of PDGF in the experiments described above,
axon-associated survival activity should remain at normal levels
and support a normal number of oligodendrocytes, as observed. One
strong candidate for an axon-associated survival signal is
NRG/GGF (see below).
back to index
Fibroblast growth factor
The fibroblast growth factor (FGF) family has
more than twenty known members to date. They all appear to exert
their effects through four tyrosine kinase receptors,
FGFR1-FGFR4. Rat oligodendrocyte lineage cells express FGFR1,
FGFR2 and FGFR3, though their relative abundance changes as the
cells mature from early O4— progenitor to GC+
oligodendrocyte; FGFR3 is expressed most strongly in O4+
pro-oligodendrocytes, while FGFR2 is expressed only by GC+
oligodendrocytes (Bansal et al., 1996). FGFR1 is expressed at all
stages of the lineage but most strongly in mature
oligodendrocytes (Bansal et al., 1996). Oligodendrocyte lineage
cells respond to FGF in a developmental stage-specific way (see
below) and this might partly result from their stage-specific
complement of receptors.
FGF2 is a mitogen for O4+ rat
pro-oligodendrocytes, and prevents their maturation to GC+
oligodendrocytes in vitro (Gard and Pfeiffer, 1993). When
cultured in a combination of FGF2 and PDGF, early progenitors
continue to divide indefinitely as O4— cells and
further differentiation is blocked (Bögler et al., 1990). FGF2
also causes differentiated oligodendrocytes to re-express the O4
antigen, down-regulate myelin genes, change shape and synthesize
DNA without entering mitosis (Bansal and Pfeiffer, 1997). This is
not simply reversion to pro-oligodendrocyte, as with NRG/GGF (see
below), but conversion to a novel phenotype. Intraventricular
injection of FGF2 into perinatal rats caused an increase in the
number of O4+ pro-oligodendrocytes concomitant with a
reduction in the number of myelinating oligodendrocytes,
reductions in myelin protein and mRNA expression and
morphological changes to myelin sheaths, consistent with the in
vitro observations (Goddard et al., 1999).
It is often stated that FGF up-regulates PDGFRa in early
progenitor cells, implying that this augments their
responsiveness to PDGF and accounts for the prolonged
proliferation and inhibition of differentiation observed when
progenitors are cultured in the presence of FGF plus PDGF
(Bögler et al., 1990). The crucial experimental observation is
that the level of PDGFRa mRNA is higher in cultures of O-2A progenitors
maintained in FGF2 plus PDGF than it is in parallel cultures
maintained in PDGF alone (McKinnon et al., 1990). However, this
does not distinguish cause and effect; it could be that the
primary effect of FGF is to inhibit differentiation, thus
maintaining (rather than up-regulating) PDGFRa expression in
progenitor cells – it is known that PDGFRa is rapidly
down-regulated once O-2A progenitors stop dividing and
differentiate (Hart et al., 1989; Butt et al., 1997a). Hence, one
can not necessarily conclude that FGF directly controls PDGFRa gene
expression, or that the synergy displayed by FGF and PDGF results
from an enhanced PDGF-driven response.
We do not have any idea as yet which of the
large number of potential FGF ligands might act on
oligodendrocyte lineage cells in vivo. There is likely to be
redundancy among FGF ligands in any case so identifying those
with an in vivo role will be difficult. Some obvious candidates
– e.g. FGF1 (acidic FGF) and FGF2 (basic FGF) – have
been deleted in mice with no obvious deleterious effects,
although close examination of the FGF2 null revealed defects
including reduced numbers of neurons in most layers of the
neocortex (Ortega et al., 1998; Miller et al., 2000). The FGF1/FGF2
double knockout has no additional defects (Miller et al., 2000). FGF3-FGF10
have also been targeted in mice; most of these are either
viable with no dysmyelinating phenotype (FGF3, FGF5, FGF6,
FGF7), or else they die very early in utero (FGF4, FGF8,
FGF9) (Mansour et al., 1993; Hebert et al., 1994; Feldman et
al., 1995; Guo et al., 1996; Fiore et al., 1997; Meyers et al.,
1998; D. Ornitz, personal communication). The FGF10 null
mouse dies at birth from respiratory failure (Min et al., 1998;
Sekine et al., 1999); oligodendrogenesis has not yet been
examined. The receptor knockouts might be expected to be
informative. However, both FGFR1 and FGFR2 null
mutations are embryonic lethal (Deng et al., 1994; Yamaguchi et
al., 1994; Deng et al., 1997) while the FGFR3 null has
abnormal bone growth and is deaf but has no dysmyelinating
phenotype (Colvin et al., 1996; Wang et al., 1999). Clearly, we
still have some way to go before the precise roles of FGF
signalling in vivo are established.
back
to index
Neurotrophins
Neurotrophin-3 (NT-3) stimulates BrdU
incorporation in O-2A progenitors and pro-oligodendrocytes from
rat optic nerve, and promotes survival of GC+
oligodendrocytes in vitro (Barres et al., 1993a; Barres et
al., 1994a; Cohen et al., 1996; Kumar et al., 1998). Although
nerve growth factor (NGF) has no mitogenic effect on its own, it
can potentiate the mitogenic effect of FGF (but not PDGF) and
also promotes survival of differentiated oligodendrocytes (Cohen
et al., 1996). In keeping with these observations, progenitor
cells and oligodendrocytes from rat brain express TrkA and TrkC,
the high affinity receptors for NGF and NT-3, respectively (NT-3
can also bind at lower affinity to certain TrkA isoforms) (Barres
et al., 1994a; Cohen et al., 1996). The low-affinity neurotrophin
receptor p75 and a truncated TrkB receptor that lacks the
tyrosine kinase domain are also expressed at low levels in
progenitors and are up-regulated during oligodendrocyte
differentiation (Cohen et al., 1996). NT-3 and related
neurotrophins are expressed by many neurons in the CNS (Ernfors
et al., 1990), so NT-3 could mediate neuron-oligodendrocyte
interactions in vivo. Note that Robinson and Miller (1997) found
that although optic nerve progenitors were TrkC immunoreactive
and responsive to NT-3, spinal cord progenitors were not,
indicating that there is regional variation in the response to
NT-3 (also see Ibarrola et al., 1996).
NT-3 influences oligodendrocyte development in
vivo. When exogenous NT-3 was delivered to the developing rat
optic nerve in vivo, there was a significant increase in the
number of oligodendrocyte progenitors and oligodendrocytes in the
nerve (Barres et al., 1994a). Conversely, when an anti-NT-3
neutralizing antibody was delivered to the nerve over a period of
several days in vivo, the numbers of oligodendrocyte lineage
cells were reduced (Barres et al., 1994a). Consistent these
findings, there is a ~30% reduction in the number of PDGFRA+
progenitors in the spinal cords of NT-3 knockout mice and
a ~15% reduction in TrkC knockouts (Kahn et al., 1999).
The disparity between receptor and ligand knockouts possibly
indicates that NT-3 exerts part of its effect through TrkA
or another receptor in vivo. There were also modest reductions in
the number of MBP+ and GC+
oligodendrocytes in the neonatal spinal cord; since both
knockouts die shortly after birth it is difficult to look later
than this. The cross-sectional area of the spinal cord is reduced
by ~30-40% in the NT-3 null mice and by somewhat less in
the TrkC null (Kahn et al., 1999), so some of the
reduction in oligodendrocyte lineage cells could be caused
indirectly by the loss of neurons, astrocytes or other cells in
the cord. Overall, the evidence indicates that NT-3 plays a
significant, though perhaps not a critical role in
oligodendrocyte progenitor cell proliferation in vivo. The role
of neurotrophins in oligodendrocyte survival remains to be fully
explored in vivo, perhaps using cell type-specific or inducible
knockout mice.
back to index
Insulin and insulin-like growth factors
Oligodendrocytes and their progenitors express
both insulin receptors and insulin-like growth factor I (IGF-1)
receptors (McMorris and Dubois-Dalcq, 1988; Baron-Van Evercooren
et al., 1991). It is not clear whether insulin itself is
available in the developing CNS but IGF-1 is expressed by
astrocytes (Ballotti et al., 1987; Ayer-le Lievre et al., 1991)
and possibly other cells. The effect of adding IGF-1 to cultures
containing oligodendrocyte lineage cells is to increase the
number of differentiated, GC+ oligodendrocytes
(McMorris et al., 1986; McMorris and Dubois-Dalcq, 1988). Part of
the effect could be due to increased progenitor cell
proliferation, since IGF-1 has been shown to be a necessary
mitogenic co-factor for FGF and NT-3 (Barres et al., 1993a;
Barres and Raff, 1994). However, IGF-1 also enhances
oligodendrocyte survival and this is probably the major effect
(Barres et al., 1993a). For example, IGF-1 promotes survival of
immuno-purified oligodendrocytes in culture and delivering
exogenous IGF-1 to the developing rat optic nerve prevents most
of the naturally-occurring death among newly-formed
oligodendrocytes (Barres et al., 1993b). Moreover,
intraventricular injection of IGF-1 leads to an increase in the
number of myelinating oligodendrocytes in the postnatal AMV
(Goddard et al., 1999). Transgenic mice that over-express IGF-1
constitutively under the control of the metallothionine promoter
have brains that are 50% heavier than normal, increased numbers
of oligodendrocytes, more myelin per oligodendrocyte and about
twice the total weight of myelin (Carson et al., 1993).
Conversely, transgenic mice that over-express the inhibitory IGF
binding protein IGFBP-1 have about half the normal weight of
myelin (Ye et al., 1995). In principle, therefore, IGF-1 could be
an important regulator of oligodendrogenesis and myelination in
vivo.
IGF-1 knockout mice have smaller brains
than normal mice, but brain weight is not reduced in proportion
to the reduction of total body weight (~30% versus ~60%,
respectively) (Beck et al., 1995). There are no neurological
signs of dysmyelination and, although IGF-1 null brains
have less myelin than wild type brains, the reduction is in
proportion to the reduced brain weight and the reduced number of
axons (Cheng et al., 1998). An exception is the optic bulb where
there is a disproportionate lack of myelin (Cheng et al., 1998).
Therefore, though IGF-1 has an important influence on
oligodendrocyte survival and myelin synthesis in vivo this might
be indirect, through IGF-mediated effects on neuronal growth and
survival. The overriding influence on oligodendrocyte number
seems to be an axon-associated factor(s), which in most CNS
regions seems not to be IGF-1. As discussed below, a likely
candidate is NRG/GGF.
back to index
Neuregulin/glial growth factor
It has been known for a long time that axon
membrane (axolemma) preparations are mitogenic for Schwann cells
in culture, and this was presumed to reflect the presence of a
growth factor(s) bound to the axonal surface (Wood and Bunge,
1975). Early attempts to identify mitogens for Schwann cells led
to the identification of an activity in pituitary extracts that
was called glial growth factor (GGF) (Raff et al., 1978; Lemke
and Brockes, 1984). When the molecule responsible was cloned it
was recognized as a member of the neuregulin (NRG) growth factor
family (Marchionni et al., 1993). The NRG family includes both
membrane-associated and diffusible factors that are the products
of three genes in mammals, NRG1, -2 and -3,
each of which encodes several alternative-splice products. GGF is
one such product of the NRG1 gene; others include the neu
differentiation factors (NDFs), acetylcholine receptor-inducing
activity (ARIA), sensory and motor neuron-derived factor (SMDF)
and the heregulins (for reviews see Burden and Yarden, 1997;
Adlkofer and Lai, 2000). Here, I refer to all NRG1 isoforms
collectively as NRG/GGF. A major portion of the axon-associated
Schwann cell mitogenic activity has been ascribed to NRG/GGF, and
it is now clear that it is important for oligodendrocyte lineage
development too.
The NRGs all exert their effects through the
ErbB family of tyrosine kinase receptors, ErbB1-ErbB4, (also
called HER1-HER4). ErbB3 and ErbB4 are direct binding partners
for NRG/GGF while ErbB1 (the epidermal growth factor receptor)
and ErbB2 are co-receptors that are recruited by ligand-bound
ErbB3 or ErbB4. Canoll et al. (1996) and Raabe et al. (1997)
found that primary O4+ pro-oligodendrocytes and GC+
oligodendrocytes express ErbB2, -B3 and -B4, ErbB3 being the most
abundant, whereas Vartanian et al. (1997) found only ErbB2 and
-B4 on these cells. This discrepancy probably reflects
differences in the culture conditions or developmental stage of
the cells being studied. RNA analyses indicate that erbB4
is most abundant in the embryonic CNS, while erbB3 comes
up during early postnatal development. Since the ErbB receptors
dimerize in various combinations and each dimer pair could
mediate a different subset of biological responses, it is
possible that NRG/GGF might affect oligodendrocyte lineage cells
in different ways at each stage of their progression from early
precursor to myelinating cell.
Vartanian et al. (1999) reported that
development of O4+ oligodendrocytes in explant
cultures of embryonic mouse spinal cord requires NRG/GGF. They
found that oligodendrocytes did not appear in explants of ventral
spinal cord from NRG/GGF null mice, but the cultures could
be "rescued" by adding pure NRG/GGF to the medium.
Conversely, oligodendrocyte development in wild type explants
could be blocked by NRG/GGF inhibitors. However, since O4 is a
relatively late lineage marker in mice (Fanarraga et al., 1995),
it is not clear from these experiments whether NRG/GGF is
required early or late during oligodendrogenesis.
Some investigators have found NRG/GGF to be a
potent mitogen for rat oligodendrocyte progenitors, particularly
O4+ pro-oligodendrocytes (Canoll et al., 1996; Canoll
et al., 1999), whereas other studies failed to find a mitogenic
effect (Vartanian et al., 1994; Raabe et al., 1997). Shi et al.
(1998) found that NRG/GGF by itself is not strongly mitogenic for
purified perinatal or adult O-2A progenitors but requires
intracellular cAMP levels to be raised (e.g. by forskolin) as
well as the input of other factors such as PDGF. It seems likely
that differences in the observed mitogenic effects of NRG/GGF
might reflect differences in the culture conditions or the
source, purity or state of differentiation of the cells –
which could influence their repertoire of ErbB receptors, for
example. Most workers in the field are agreed that NRG/GGF is a
potent survival factor for differentiated oligodendrocytes,
however (Canoll et al., 1996; Raabe et al., 1997; Canoll et al.,
1999).
Apart from its mitogenic and survival-promoting
effects, NRG/GGF has been found to arrest lineage progression at
the pro-oligodendrocyte stage in vitro, preventing them from
differentiating further to GC+ oligodendrocytes
(Canoll et al., 1996). Conversely, NRG/GGF was found to induce
phenotypic reversion of GC+ oligodendrocytes to O4+
pro-oligodendrocytes, causing them to re-enter the cell division
cycle and proliferate in vitro (Canoll et al., 1999). This could
be important in the context of demyelination/remyelination during
multiple sclerosis and other demyelinating diseases.
NRG/GGF is expressed by some neuroepithelial
cells in the embryonic spinal cord and brain, by cells in the
forebrain SVZ and by a variety of differentiated neurons
(Orr-Urtreger et al., 1993; Corfas et al., 1995; Meyer et al.,
1997; Vartanian et al., 1999). The expression pattern is
therefore consistent with NRG/GGF playing both early and late
roles in oligodendrocyte lineage progression. Mice that are
homozygous null for NRG1, erbB2, erbB3 and erbB4
all demonstrate a requirement for NRG/ErbB signalling in
formation of the peripheral nervous system and the heart
(Gassmann et al., 1995; Lee et al., 1995; Meyer and Birchmeier,
1995; Kramer et al., 1996; Riethmacher et al., 1997). They die
mid-gestation, precluding analysis of oligodendrogenesis in vivo.
However, it has recently proved possible to prolong the life of
at least one of these knockout mice by expressing the missing
gene product(s) under the control of a heart-specific gene
promoter (Woldeyesus et al., 1999). This should allow analysis of
later functions of the NRG/ErbB signalling system including its
role in the CNS.
back to index
Chemokine GRO-a
Recently, the growth-regulated oncogene-a (GRO-a ), a human CXC
chemokine, has entered the fray as a mitogenic co-factor for
oligodendrocyte progenitors. This factor is homologous to KC, a
PDGF-induced immediate early gene product in NIH-3T3 cells (i.e.
it is transcriptionally activated by PDGF in 3T3 cells in the
absence of new protein synthesis; Cochran et al., 1983). GRO-a peptides are
involved in growth regulation of a variety of cell lineages
including fibroblasts and hemopoeitic precursors. CXC chemokines
including GRO-a are up-regulated in the CNS during
experimentally-induced demyelination (Glabinski et al., 1998) and
in the dysmyelinating mouse mutant jimpy (Wu et al.,
2000).
As mentioned before, PDGF is not by itself
strongly mitogenic for purified O-2A progenitor cells in culture.
The mitogenic response of progenitors to PDGF is greatly
potentiated by factors present in mixed cell cultures of spinal
cord or in medium conditioned by spinal cord cultures (Robinson
and Miller, 1996); it now seems that a large part of this
potentiating activity is due to GRO-a (Robinson et al., 1998).
The polypeptide by itself is not mitogenic but it enhances BrdU
incorporation in O4— early progenitors
several-fold over that observed with PDGF alone. The enhancement
is only observed with saturating concentrations (10 ng/ml)
of PDGF and is optimum only over a narrow range of GRO-a concentrations
(Robinson et al., 1998). There is no mitogenic effect on O4+
pro-oligodendrocytes. A potential source of GRO-a in vivo is
astrocytes, since cultured astrocytes secret GRO-a and immunoreactive
GRO-a
can be detected in white matter glia, probably astrocytes, in
situ (Robinson et al., 1998). It was suggested that transient
synergy between PDGF and GRO-a might stimulate a local burst of progenitor cell
proliferation, such as the late burst that is thought to occur in
white matter when progenitors have stopped migrating and before
they finally differentiate into oligodendrocytes (Robinson et
al., 1998).
back to index
Other factors involved in oligodendrocyte
proliferation or survival
A variety of other diffusible factors have been
implicated in the regulation of proliferation and/or survival in
the oligodendrocyte lineage. These include the cytokines ciliary
neurotrophic factor (CNTF), interleukin-6 (IL-6) or leukemia
inhibitory factor (LIF), all of which enhance oligodendrocyte
survival in culture (Barres et al., 1993a; Mayer et al., 1994;
Vos et al., 1996). The survival-promoting activities of
cytokines, neurotrophins and insulin/IGF-1/IGF-2 are additive, so
that oligodendrocytes can be kept alive for weeks in a
combination of factors including at least one member of each of
these groups of molecules (Barres et al., 1993a). Delivery of
exogenous CNTF has been shown to promote oligodendrocyte
development in the optic nerve in vivo (Barres et al., 1993a;
Barres et al., 1996), apparently by stimulating progenitor cell
proliferation, since CNTF has been shown to enhance PDGF-driven
progenitor cell proliferation in vitro (Barres et al., 1996).
Conversely, progenitor cell proliferation is reduced and
oligodendrocyte production retarded in transgenic mice lacking
CNTF, although myelination is ultimately normal (Barres et al.,
1996). Therefore, CNTF plays a non-essential role in
oligodendrocyte development. LIF also plays a subtle role because
a reduction in MBP immunoreactivity in the brains of female (not
male) LIF knockout mice has been reported (Bugga et al., 1998).
Apart from the diffusible factors discussed
above, several other classes of molecules are also important for
oligodendrocyte lineage development. Interactions between the
cell and extracellular matrix are known to provide a mitogenic
input, sometimes in synergy with growth factors. For example,
oligodendrocyte lineage cells express an evolving set of a and b integrin subunits
as they mature and these appear to influence not only
cell-substrate adhesion and migration, but also proliferation,
differentiation and survival (Frost et al., 1999; Blaschuk et
al., 2000). Non-peptide agents such as thyroid hormones, retinoic
acid and progesterone have also been implicated in
oligodendrocyte development. Of particular note are the thyroid
hormones (TH), which have been implicated in the proliferation of
PSA-NCAM+ neuroepithelial glial precursors (Ben-Hur et
al., 1998) and which are also required later for progenitor cells
to differentiate into oligodendrocytes (see below). Finally,
there is a growing awareness of the role of neurotransmitters and
ion channels in oligodendrocyte proliferation control; this is
the subject of Chapter X (Gallo).
back to index
III Controls on oligodendrocyte
differentiation
A cell division limiter in oligodendrocyte
progenitors; the role of thyroid hormones
When a single oligodendrocyte progenitor is
cultured on its own in defined medium in the absence of other
cells or added mitogens, it stops dividing and differentiates
within a day or two into an oligodendrocyte. This suggested that
oligodendrocyte differentiation is a default pathway that does
not require signals from other cells. However, it turns out that
the thyroid hormone triiodothyronine (T3), a constituent of the
defined medium used for the early experiments (Bottenstein and
Sato, 1979) is important for timely oligodendrocyte
differentiation in vitro.
When perinatal optic nerve O-2A progenitors are
cultured in mitogens such as PDGF, they proliferate for a time
but not indefinitely; eventually they stop dividing and, as long
as TH is present in the medium, they differentiate. If there are
survival factors present they survive and mature, otherwise they
die. What makes the progenitors stop dividing? The cells seem to
have a built-in division limiter, or "clock". The
evidence for this is as follows. If a single perinatal optic
nerve progenitor is placed in a microwell in the presence of
mitogens (e.g. astrocyte-conditioned medium) it divides a few
times then all its progeny differentiate more-or-less
synchronously into oligodendrocytes (Temple and Raff, 1985). A
given progenitor can go through any number of divisions between
zero and approximately eight. However, if the two daughters of
the first division are separated and transferred to different
microwells, both siblings usually go though the same number of
further divisions before differentiating (Temple and Raff, 1986).
It seems that each progenitor remembers its mitotic history, even
in isolation, and retires from the cell division cycle after a
pre-set time or number of divisions. The fact that not all
progenitors go through the same number of divisions in this
experiment is presumed to be because they have already used up a
variable amount of their time or divisions in vivo, before the
beginning of the experiment. This implies that new migratory
progenitor cells must be produced continuously within the VZ (the
part that supplies the optic nerve, at least) over an extended
period of time. It is not known if this is correct but it might
be testable.
A role for TH in these timing events was
suggested by the observation that when T3 was omitted from the
culture medium, the progenitor cells seemed to divide
indefinitely in response to PDGF or NT-3 (Barres et al., 1994b).
In fact they do not go on for ever but eventually stop dividing
even in the absence of TH; nonetheless, the normal limit on their
proliferation is greatly extended (Gao et al., 1998). It appears
that TH is not required for timing per se, but rather for
triggering cell cycle exit at the appropriate time; if
progenitors are cultured in the absence of TH beyond the time
that they would normally differentiate and then TH is added
belatedly, the cells differentiate more rapidly and synchronously
than they would have done in the continuous presence of the
hormone (Barres et al., 1994b). It is as if they remember that
they have exceeded the division limit (i.e. the intrinsic timer
still works) but they are unable to exit the cell cycle unless TH
is present. This "effector" function of TH can be
mimicked by glucocorticoids or retinoic acid (RA) (Barres et al.,
1994b).
There is an established literature on the role
of TH in brain development and myelination (for review see
Rodriguez-Peña, 2000). For example, the start of myelination is
delayed in hypothyroid rats (Balazs et al., 1969; Walters and
Morell, 1981; Ibarrola and Rodriguez-Peña, 1997) and accelerated
in hyperthyroid rats (Marta et al., 1998) or rats that receive
postnatal injections of T3 (Barres et al., 1994b). Moreover, TH
normally only becomes available in the rat when the thyroid gland
becomes active after birth, which is around the time myelin first
starts to appear. Oligodendrocytes and their progenitors possess
receptors for T3 (Baas et al., 1994; Fierro-Renoy, 1995; Gao et
al., 1998; Carre et al., 1998) so it is likely that T3 acts
directly on oligodendrocyte lineage cells to control their
differentiation in vivo.
back to index
Cell-intrinsic and cell-extrinsic controls
on progenitor cell proliferation and population growth
How does the cell-intrinsic division limiter
(timer) work? There is evidence that inhibitors of
cyclin-dependent kinases (cdks) - in particular, p27Kip1
- are key. As progenitor cells age in culture the amount of
immunoreactive p27Kip1 in their nuclei increases and
remains high after they differentiate into oligodendrocytes
(Durand et al., 1997). This suggests a model in which p27Kip1
accumulates with time until eventually a critical threshold is
reached, triggering cell cycle exit and oligodendrocyte
differentiation. Consistent with this, p27Kip1 null
mice are about 20% larger than normal, suggesting that progenitor
cells of all sorts go through more divisions than normal in the
absence of p27Kip1. Moreover, oligodendrocyte
progenitors from optic nerves of p27Kip1 null mice
undergo more divisions than normal in vitro (Durand et al., 1998)
and oligodendrocyte differentiation is perturbed
(Casaccia-Bonnefil et al., 1997). Other candidate components of
the cell-intrinsic timing mechanism are TH receptors (b isoforms) (Barres
et al., 1994b; Gao et al., 1998), the transcription factor
SCIP/Oct-6 (Collarini et al., 1992) and the helix-loop-helix
protein ID4 (Kondo and Raff, 2000).
A strict cell-intrinsic timing mechanism might
not be adequate to explain the time course of oligodendrogenesis
in all parts of the CNS. Cell clones containing both
oligodendrocyte progenitors and differentiated oligodendrocytes
commonly develop in low density spinal cord cultures, indicating
that the progeny of single precursor cells do not necessarily
differentiate synchronously as first described for optic nerve
progenitors (Zhang and Miller, 1995; Ibarrola et al., 1996). In
the spinal cord, oligodendrocytes continue to be generated for
several weeks after birth, implying - if a cell-intrinsic timer
were solely responsible for timing differentiation - that new
progenitor cells must be generated continuously in the VZ over a
similar extended period. This is possible but seems unlikely
since oligodendrocyte lineage markers do not persist that long in
the spinal cord VZ. - for example, olig-1 expression is
detected in the ventral VZ for only a few days before birth (Lu
et al., 2000; N. Tekki-Kessaris and WDR, unpublished
observations).
Another type of observation indicates that
progenitor cell proliferation is not always determined purely
from within the cell. By inter-breeding two independent lines of NSE-PDGF-A
transgenic mice we have been able to generate offspring with up
to four transgene loci and a large (up to ~20-fold) increase in
the expression of PDGF-A transcripts (van Heyningen et
al., 2000). If the number of progenitor cell divisions were
limited by a cell-autonomous mechanism, we might expect
progenitor cell numbers to saturate at some point, regardless of
transgene dose and amount of PDGF available. However, we found
that the number of progenitor cells continued to increase in
proportion to the level of PDGF-A mRNA (which presumably
correlates with the rate of PDGF protein synthesis) until there
were around twenty times the normal number of progenitors before
birth – and no sign of an approaching plateau (Calver et
al., 1998; van Heyningen et al., 2000). At this point the
new-born animals were no longer viable. At the other extreme, PDGF-A
(-/-) embryos had very few progenitors indeed (<10% wild type)
and PDGF-A (+/-) littermates had half of the wild type
number. This demonstrates vividly that progenitor cell numbers in
the cord are not determined by cell-intrinsic mechanisms - at
least not to an extent that is useful to the animal - but rather
are set by rates of supply of extracellular mitogens such as
PDGF.
Thus, there is evidence that the progenitor
cell proliferation is influenced both by cell-intrinsic and
cell-extrinsic controls on cell division. This is to be expected,
given that cell cycle control proteins (e.g. cdks, cdk
inhibitors) are controlled by factors outside the cell and,
conversely, cdks and cdk inhibitors modulate the cell’s
response to extracellular factors. While there clearly are
intracellular mechanisms that limit a cell's capacity to
proliferate in vitro, they do not necessarily override
environmental controls that operate in vivo. From experiments
quoted above it seems likely that the steady-state number of
progenitor cells and the time of onset of oligodendrocyte
maturation in the embryonic and early postnatal spinal cord is
influenced as much (or more) by limiting amounts of extracellular
factors (e.g. PDGF and TH) as by cell-autonomous mechanisms.
However, the intracellular timer is clearly present and might
kick in to terminate cell proliferation later in development -
for example in the optic nerve, which is an inherently
late-developing part of the CNS. There is also evidence that
long-term changes in the properties and behaviour of progenitor
cells (e.g. progenitor cells in the adult versus those in the
perinatal CNS) are driven by cell-intrinsic mechanisms (Gao and
Raff, 1997; Tang et al., 2000)
There is also evidence that the Notch
signalling pathway plays a role in timing oligodendrocyte
differentiation. Oligodendrocytes express Notch1 and their
differentiation is strongly inhibited in vitro by Notch ligands
Jagged or Delta (Wang et al., 1998). Notch1 is expressed on optic
nerve oligodendrocytes and its ligand Jagged-1 on retinal
ganglion cell axons; neuronal expression of Jagged-1 declines
during the period of active myelination in vivo, consistent with
a role in timing oligodendrocyte differentiation (Wang et al.,
1998). An attractive idea is that down-regulation of Jagged might
correlate with when axons reach their targets and establish
synaptic contacts, thus preventing myelination of growing axons.
A picture is emerging in which cell contact-mediated signals
(e.g. Jagged, NRG/GGF) combine with short-range paracrine signals
(e.g. PDGF, FGF) and long-range systemic signals (e.g. TH,
retinoic acid) to control oligodendrocyte differentiation and
maturation in a way that serves the requirements of both the
local environment and the organism as a whole.
back to index
Conclusion and Outstanding questions
There is a plethora of signalling molecules
that influence development of the oligodendrocyte lineage from
specification to myelination (Fig. 5). However, while there
is an intimidating amount of information available, there are
still fundamental questions to which we need answers.
There are still uncertainties about the sites
of origin of oligodendrocyte precursors in the germinal zones,
particularly of the brain. We need to know where and when the
lineage is specified if we are to stand a chance of discovering
how they are specified. We also need to make a major effort to
understand how precursor cells defined by different assays or
isolated from different regions of the CNS relate to one another
– tripotential GRPs in the embryonic spinal cord, PSA-NCAM+
precursors in the embryonic brain, pluripotent precursors in the
postnatal and adult CNS and, of course, how these all relate to
the familiar O-2A progenitor cell.
It is noteworthy that several of the factors we
have discussed seem to influence the oligodendrocyte lineage in
stage-specific ways. A clear example is TH, which influences
proliferation of PSA-NCAM+ pre-progenitor cells as
well as controlling later events concerned with cell cycle exit
and oligodendrocyte maturation. This suggests that cells'
responses to a given factor changes with their state of maturity
– whether this be through changing repertoires of receptors
or different downstream signal transduction molecules is unknown.
Clearly there is a need to learn more about intracellular
signalling, a subject which has not been broached in this
Chapter. The changing responses of cells to their environment is
a general developmental issue that might be illuminated by
studying the oligodendrocyte lineage.
We do not understand enough about the complex
dynamics of how a population of progenitor cells can generate
differentiated, post-mitotic progeny while continuing to divide
and maintain the progenitor pool. For example, there is still a
lot of uncertainty about the scale of cell death. What is the
clearance rate of dead cells and does this vary according to area
– grey versus white matter, for example? Knowing clearance
rates accurately would allow us to calculate death rates with
more confidence. Do progenitor cells die, or do they always start
to differentiate before dying as immature oligodendrocytes? What
is relationship between proliferation and migration? New methods
of imaging cells in vivo (in zebrafish, for example) or in
explant cultures might allow us to follow cell division,
migration,. differentiation and death in real time. How important
is cell-autonomous behaviour compared to the influence of the
extracellular environment? To answer this we need to know more
about the local environment that oligodendrocyte lineage cells
experience in vivo - the local concentrations of growth factors
and so on - and also we need more detailed information about how
the cells actually behave in vivo. For this we need to get back
to basics and measure, for example, stage- and region-specific
rates of cell division in vivo.
Crucially, we need to test more of our ideas in
an in vivo setting - in transgenic animals, for example. This is
difficult, time-consuming and expensive but the promise is that
by gaining a thorough understanding of oligodendrocyte
development in vivo we will come to discover more about
development (and Biology) in general and, in doing so, learn how
to manipulate the clinically important oligodendrocyte lineage to
the future benefit of individuals with dysmyelinating disease.
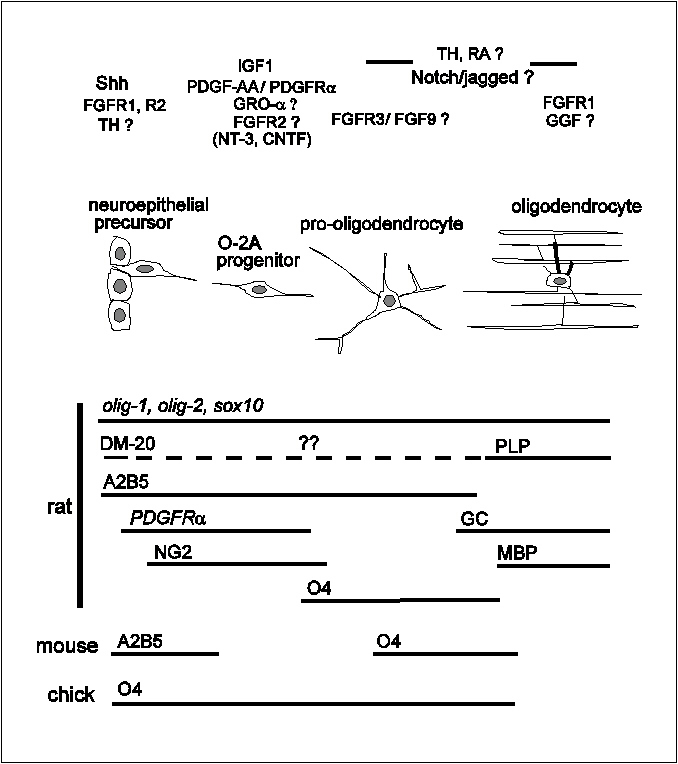 Figure 5 Summary diagram of oligodendrocyte
lineage progression. Shown below the diagram are lineage markers
that have been used to identify various stages of the lineage,
mainly in rat. Differences in the specificity of markers A2B5 and
O4 are indicated for mouse and chick. Above the diagram are
listed some of the signalling molecules that are thought to
influence the lineage as it develops. For details see the text. back to index
Figure 5 Summary diagram of oligodendrocyte
lineage progression. Shown below the diagram are lineage markers
that have been used to identify various stages of the lineage,
mainly in rat. Differences in the specificity of markers A2B5 and
O4 are indicated for mouse and chick. Above the diagram are
listed some of the signalling molecules that are thought to
influence the lineage as it develops. For details see the text. back to index
_________________________________________________________________________________________
Acknowledgements
The author thanks members of his laboratory and
many others, too numerous to name here, for ideas, discussions
and helpful comments. Work in the author's laboratory is funded
by the Medical Research Council and the Wellcome Trust.
back to top
back to index
References
Adlkofer, K.
and Lai, C. (2000). Role of neuregulins in glial cell
development. Glia 29, 104-11.
Ayer-le
Lievre, C., Stahlbom, P.-A., and Sara, V.R. (1991). Expression of
IGF-I and -II mRNA in the brain and craniofacial region of the
rat fetus. Development 111, 105-15.
Baas, D.,
Fressinaud, C., Ittel, M.E., Reeber, A., Dalencon, D., Puymirat,
J., and Sarlieve, L.L. (1994). Expression of thyroid receptor
isoforms in rat oligodendrocyte cultures. Neuroscience Letters
176, 47-51.
Balazs, R.,
Brooksbank.B.W., Davison, A.N., Eayrs, J.T., and Wilson, D.A.
(1969). The effect of neonatal thyroidectomy on myelination in
the rat brain. Brain Research 15, 219-32.
Ballotti, R.,
Nielsen, F.C., Pringle, N., Kowalski, A., Richardson, W.D., Van
Obberghen, E., and Gammeltoft, S. (1987). Insulin-like growth
factor I in cultured rat astrocytes: expression of the gene and
receptor tyrosine kinase. EMBO Journal 6, 3633-9.
Bansal, R.
and Pfeiffer, S.E. (1992). Novel stage in the oligodendrocyte
lineage defined by reactivity of progenitors with R-mAb prior to
O1 galactocerebroside. Journal of Neuroscience Research 32,
309-16.
Bansal, R.,
Kumar, M., Murray, K., Morrison, R.S., and Pfeiffer, S.E. (1996).
Regulation of FGF receptors in the oligodendrocyte lineage.
Molecular andCellular Neuroscience 7, 263-75.
Bansal, R.
and Pfeiffer, S.E. (1997). FGF-2 converts mature oligodendrocytes
to a novel phenotype. Journal of Neuroscience Research 50,
215-28.
Baron-Van
Evercooren, A., Olichon-Berthe, C., Kowalski, A., Visciano, G.,
and Van-Obberghen, E. (1991). Expression of IGF-I and insulin
receptor genes in the rat central nervous system: a
developmental, regional, and cellular analysis. Journal of
Neuroscience Research 28, 244-53.
Barres, B.A.,
Hart, I.K., Coles, H.S.R., Burne, J.F., Voyvodic, J.T.,
Richardson, W.D., and Raff, M.C. (1992). Cell death and control
of cell survival in the oligodendrocyte lineage. Cell 70,
31-46.
Barres, B.A.
and Raff, M.C. (1993). Proliferation of oligodendrocyte precursor
cells depends on electrical activity in axons. Nature 361,
258-60.
Barres, B.A.,
Schmid, R., Sendnter, M., and Raff, M.C. (1993a). Multiple
extracellular signals are required for long-term oligodendrocyte
survival. Development 118, 283-95.
Barres, B.A.,
Jacobson, M.D., Schmid, R., Sendtner, M., and Raff, M.C. (1993b).
Does oligodendrocyte survival depend on axons? Current Biology
3, 489-97.
Barres, B.A.
and Raff, M.C. (1994). Control of oligodendrocyte number in the
developing rat optic nerve. Neuron 12, 935-42.
Barres, B.A.,
Raff, M.C., Gaese, F., Bartke, I., Dechant, G., and Barde, Y.A.
(1994a). A crucial role for neurotrophin-3 in oligodendrocyte
development. Nature 367, 371-5.
Barres, B.A.,
Lazar, M.A., and Raff, M.C. (1994b). A novel role for thyroid
hormone, glucocorticoids and retinoic acid in oligodendrocyte
development. Development 120, 1097-108.
Barres, B.A.,
Burne, J.F., Holtmann, B., Thoenen, H., Sendtner, M., and Raff,
M.C. (1996). Ciliary neurotrophic factor enhances the rate of
oligodendrocyte generation. Molecular and Cellular
Neuroscience 8, 146-56.
Basler, K.,
Edlund, T., Jessell, T.M., and Yamada, T. (1993). Control of cell
pattern in the neural tube: regulation of cell differentiation by
dorsalin-1, a novel TGF beta family member. Cell 73,
687-702.
Beck, K.D.,
Powell-Braxton, L., Widmer, H.R., Valverde, J., and Hefti, F.
(1995). Igf1 gene disruption results in reduced brain size, CNS
hypomyelination, and loss of hippocampal granule and striatal
parvalbumin-containing neurons. Neuron 14, 717-30.
Ben-Hur, T.,
Rogister, B., Murray, K., Rougon, G., and Dubois-Dalcq, M.
(1998). Growth and fate of PSA-NCAM+ precursors of the postnatal
brain. Journal of Neuroscience 18, 5777-88.
Birling, M.C.
and Price, J. (1998). A study of the potential of the embryonic
rat telencephalon to generate oligodendrocytes. Developmental
Biology 193, 100-13.
Blaschuk,
K.L., Frost, E.E., and ffrench-Constant, C. (2000). The
regulation of proliferation and differentiation in
oligodendrocyte progenitor cells by alphaV integrins. Development
127, 1961-9.
Bottenstein,
J.E. and Sato, G.H. (1979). Growth of a rat neuroblastoma cell
line in serum-free supplemented medium. Proceedings of the
National Academy of Sciences USA 76, 514-7.
Bögler, O.,
Wren, D., Barnett, S.C., Land, H., and Noble, M. (1990).
Cooperation between two growth factors promotes extended
self-renewal and inhibits differentiation of O-2A progenitor
cells. Proceedings of the National Academy of Sciences USA
87, 6368-72.
Briscoe, J.
and Ericson, J. (1999). The specification of neuronal identity by
graded Sonic Hedgehog signalling. Seminars in Cell and
Developmental Biology 10, 353-62.
Bugga, L.,
Gadient, R.A., Kwan, K., Stewart, C.L., and Patterson, P.H.
(1998). Analysis of neuronal and glial phenotypes in brains of
mice deficient in leukemia inhibitory factor. Journal of
Neurobiology 36, 509-24.
Burden, S.
and Yarden, Y. (1997). Neuregulins and their receptors: a
versatile signaling module in organogenesis and oncogenesis. Neuron
18, 847-55.
Butt, A.M.,
Hornby, M.F., Ibrahim, M., Kirvell, S., Graham, A., and Berry, M.
(1997a). PDGF-alpha receptor and myelin basic protein mRNAs are
not co-expressed by oligodendrocytes in vivo: a double in situ
hybridization study in the anterior medullary velum of the
neonatal rat. Molecular and Cellular Neuroscience 8,
311-22.
Butt, A.M.,
Hornby, M.F., Kirvell, S., and Berry, M. (1997b).
Platelet-derived growth factor delays oligodendrocyte
differentiation and axonal myelination in vivo in the anterior
medullary velum of the developing rat. Journal of Neuroscience
48, 588-96.
Calver, A.R.,
Hall, A.C., Yu, W.-P., Walsh, F.S., Heath, J.K., Betsholtz, C.,
and Richardson, W.D. (1998). Oligodendrocyte population dynamics
and the role of PDGF in vivo. Neuron 20, 869-82.
Cameron-Curry,
P. and Le Douarin, N.M. (1995). Oligodendrocyte precursors
originate from both the dorsal and the ventral parts of the
spinal cord. Neuron 15, 1299-310.
Canoll, P.D.,
Musacchio, M.A., Hardy, R., Reynolds, R., Marchionni, M.A., and
Salzer, J.L. (1996). GGF/neuregulin is a neuronal signal that
promotes the proliferation and survival and inhibits
differentiation of oligodendrocyte progenitors. Neuron 17,
229-43.
Canoll, P.D.,
Kraemer, R., Teng, K.K., Marchionni, M.A., and Salzer, J.L.
(1999). GGF/neuregulin induces a phenotypic reversion of
oligodendrocytes. Molecular and Cellular Neuroscience 13,
79-94.
Carre, J.L.,
Demerens, C., Rodriguez-Peña, A., Floch, H.H., Vincendon, G.,
and Sarlieve, L.L. (1998). Thyroid hormone receptor isoforms are
sequentially expressed in oligodendrocyte lineage cells during
rat cerebral development. Journal of Neuroscience Research
54, 584-94.
Carson, M.J.,
Behringer, R.R., Brinster, R.L., and McMorris, F.A. (1993).
Insulin-like growth factor I increases brain growth and central
nervous system myelination in transgenic mice. Neuron 10,
729-40.
Casaccia-Bonnefil,
P., Tikoo, R., Kiyokawa, H., Friedrich, V., Jr., Chao, M.V., and
Koff, A. (1997). Oligodendrocyte precursor differentiation is
perturbed in the absence of the cyclin-dependent kinase inhibitor
p27Kip1. Genes and Development 11,
2335-46.
Cheng, C.M.,
Joncas, G., Reinhardt, R.R., Farrer, R., Quarles, R., Janssen,
J., McDonald, M.P., Crawley, J.N., Powell-Braxton, L., and Bondy,
C.A. (1998). Biochemical and morphometric analyses show that
myelination in the insulin-like growth factor 1 null brain is
proportionate to its neuronal composition. Journal of
Neuroscience 18, 5673-81.
Cochran,
B.H., Reffel, A.C., and Stiles, C.D. (1983). Molecular cloning of
gene sequences regulated by platelet-derived growth factor. Cell
33, 939-47.
Cohen, R.I.,
Marmur, R., Norton, W.T., Mehler, M.F., and Kessler, J.A. (1996).
Nerve growth factor and neurotrophin-3 differentially regulate
the proliferation and survival of developing rat brain
oligodendrocytes. Journal of Neuroscience 16,
6433-42.
Collarini,
E.J., Kuhn, R., Marshall, C.J., Monuki, E.S., Lemke, G., and
Richardson, W.D. (1992). Down-regulation of the POU transcription
factor SCIP is an early event in oligodendrocyte differentiation
in vitro. Development 116, 193-200.
Colvin, J.S.,
Bohne, B.A., Harding, G.W., McEwen, D.G., and Ornitz, D.M.
(1996). Skeletal overgrowth and deafness in mice lacking
fibroblast growth factor receptor 3. Nature Genetics 12,
390-7.
Corfas, G.,
Rosen, K.M., Aratake, H., Krauss, R., and Fischbach, G.D. (1995).
Differential expression of ARIA isoforms in the rat brain. Neuron
14, 103-15.
Dahmane, N.
and Ruiz i Altaba, A. (1999). Sonic hedgehog regulates the growth
and patterning of the cerebellum. Development 126,
3089-100.
Deng, C.X.,
Wynshaw-Boris, A., Shen, M.M., Daugherty, C., Ornitz, D.M., and
Leder, P. (1994). Murine FGFR-1 is required for early
postimplantation growth and axial organization. Genes and
Development 8, 3045-57.
Deng, C.,
Bedford, M., Li, C., Xu, X., Yang, X., Dunmore, J., and Leder, P.
(1997). Fibroblast growth factor receptor-1 (FGFR-1) is essential
for normal neural tube and limb development. Developmental
Biology 185, 42-54.
Dickinson,
P.J., Fanarraga, M.L., Griffiths, I.R., Barrie, J.M., Kyriakides,
E., and Montague, P. (1996). Oligodendrocyte progenitors in the
embryonic spinal cord express DM-20. Neuropathology and
Applied Neurobiology 22, 188-98.
Durand, B.,
Gao, F.G., and Raff, M. (1997). Accumulation of the
cyclin-dependent kinase inhibitor p27/Kip1 and the timing of
oligodendrocyte development. EMBO Journal 16,
306-17.
Durand, B.,
Fero, M.L., Roberts, J.M., and Raff, M.C. (1998). p27Kip1
alters the response of cells to mitogen and is part of a
cell-intrinsic timer that arrests the cell cycle and initiates
differentiation. Current Biology 8, 431-40.
Ellison,
J.A., Scully, S.A., and de Vellis, J. (1996). Evidence for
neuronal regulation of oligodendrocyte development: cellular
localization of platelet-derived growth factor alpha-receptor and
A-chain mRNA during cerebral cortex development in the rat. Journal
of Neuroscience Research 45, 28-39.
Ericson, J.,
Morton, S., Kawakami, A., Roelink, H., and Jessell, T.M. (1996).
Two critical periods of Sonic Hedgehog signaling required for the
specification of motor neuron identity. Cell 87,
661-73.
Ericson, J.,
Rashbass, P., Schedl, A., Brenner-Morton, S., Kawakami, A., van
Heyningen, V., Jessell, T.M., and Briscoe, J. (1997). Pax6
controls progenitor cell identity and neuronal fate in response
to graded Shh signaling. Cell 90, 169-80.
Ernfors, P.,
Wetmore, C., Olson, L., and Persson, H. (1990). Identification of
cells in rat brain and peripheral tissues expressing mRNA for
members of the nerve growth factor family. Neuron 5,
511-26.
Fanarraga,
M.L., Sommer, I., and Griffiths, I.R. (1995). O-2A progenitors of
the mouse optic nerve exhibit a developmental pattern of antigen
expression different from the rat. Glia 15, 95-104.
Fanarraga,
M.L., Dickinson, P.J., Sommer, I., Montague, P., Kyriakides, E.,
and Griffiths,I.R. (1996). Evidence that some oligodendrocyte
progenitors in the developing optic pathway express the plp gene.
Glia 18, 282-92.
Feldman, B.,
Poueymirou, W., Papaioannou, V.E., DeChiara, T.M., and Goldfarb,
M. (1995). Requirement of FGF-4 for postimplantation mouse
development. Science 267, 246-9.
Fierro-Renoy,
J.F., Szuchet, S., Falcone, M., Macchia, E., and DeGroot, L.
(1995). Three different thyroid hormone receptor isoforms are
detected in pure culture of ovine oligodendrocytes. Glia 14,
1831-6.
Fiore, F.,
Planche, J., Gibier, P., Sebille, A., deLapeyriere, O., and
Birnbaum, D. (1997). Apparent normal phenotype of Fgf6-/- mice. International
Journal of Developmental Biology 41, 639-42.
[published erratum in International Journal of Developmental
Biology (1997). 41, following 958].
Frost, E.E.,
Buttery, P.C., Milner, R., and ffrench-Constant, C. (1999).
Integrins mediate a neuronal survival signal for
oligodendrocytes. Current Biology 9, 1251-4.
Fruttiger,
M., Karlsson, L., Hall, A.C., Abramsson, A., Calver, A.R.,
Boström, H., Willetts, K., Bertold, C.-H., Heath, J.K.,
Betsholtz, C., and Richardson, W.D. (1999). Defective
oligodendrocyte development and severe hypomyelination in PDGF-A
knockout mice. Development 126, 457-67.
Fruttiger,
M., Calver, A.R., and Richardson, W.D. (2000). PDGF-A is
constitutively secreted from neuronal cell bodies but not from
axons. Submitted.
Galileo,
D.S., Gray, G.E., Owens, G.C., Majors, J., and Sanes, J.R.
(1990). Neurons and glia arise from a common progenitor in
chicken optic tectum: demonstration with two retroviruses and
cell type-specific antibodies. Proceedings of the National
Academy of Sciences USA 87, 458-62.
Gao, F.B.,
Apperly, J., and Raff, M. (1998). Cell-intrinsic timers and
thyroid hormone regulate the probability of cell-cycle withdrawal
and differentiation of oligodendrocyte precursor cells. Developmental
Biology 197, 54-66.
Gao, F.B. and
Raff, M. (1997). Cell size control and a cell-intrinsic
maturation program in proliferating oligodendrocyte precursor
cells. J.Cell Biol. 138, 1367-1377.
Gard, A.L.
and Pfeiffer, S.E. (1990). Two proliferative stages of the
oligodendrocyte lineage (A2B5+O4- and O4+GalC-) under different
mitogenic control. Neuron 5, 615-25.
Gard, A.L.
and Pfeiffer, S.E. (1993). Glial cell mitogens bFGF and PDGF
differentially regulate development of O4+GalC-
oligodendrocyte progenitors. Developmental Biology 159,
618-30.
Gassmann, M.,
Casagranda, F., Orioli, D., Simon, H., Lai, C., Klein, R., and
Lemke, G. (1995). Aberrant neural and cardiac development in mice
lacking the ErbB4 neuregulin receptor. Nature 378,
390-4.
Glabinski,
A.R., Tuohy, V.K., and Ransohoff, R.M. (1998). Expression of
chemokines RANTES, MIP-1alpha and GRO-alpha correlates with
inflammation in acute experimental autoimmune encephalomyelitis. Neuroimmunomodulation
5, 166-71.
Goddard,
D.R., Berry, M., and Butt, A.M. (1999). In vivo actions of
fibroblast growth factor-2 and insulin-like growth factor-I on
oligodendrocyte development and myelination in the central
nervous system. Journal of Neuroscience Research 57,
74-85.
Goodrich,
L.V., Milenkovic, L., Higgins, K.M., and Scott, M.P. (1997).
Altered neural cell fates and medulloblastoma in mouse patched
mutants. Science 277, 1109-13.
Grinspan,
J.B. and Franceschini, B. (1995). Platelet-derived growth factor
is a survival factor for PSA-NCAM+ oligodendrocyte pre-progenitor
cells. Journal of Neuroscience Research 41, 540-51.
Grinspan,
J.B., Stern, J.L., Pustilnik, S.M., and Pleasure, D. (1990).
Cerebral white matter contains PDGF-responsive precursors to O2A
cells. Journal of Neuroscience 10, 1866-73.
Grove, E.A.,
Williams, B.P., Li, D.Q., Hajihosseini, M., Friedrich, A., and
Price, J. (1993). Multiple restricted lineages in the embryonic
rat cerebral cortex. Development 117, 553-61.
Guo, L.,
Degenstein, L., and Fuchs, E. (1996). Keratinocyte growth factor
is required for hair development but not for wound healing. Genes
and Development 10, 165-75.
Hall, A.,
Giese, N.A., and Richardson, W.D. (1996). Spinal cord
oligodendrocytes develop from ventrally-derived progenitor cells
that express PDGF alpha-receptors. Development 122,
4085-94.
Hardy, R.J.
(1997). Dorsoventral patterning and oligodendroglial
specification in the developing central nervous system. Journal
of Neuroscience Research 50, 139-145.
Hardy, R.J.
and Friedrich, V.J. (1996). Oligodendrocyte progenitors are
generated throughout the embryonic mouse brain, but differentiate
in restricted foci. Development 122, 2059-69.
Hart, I.K.,
Richardson, W.D., Bolsover, S.R., and Raff, M.C. (1989). PDGF and
intracellular signaling in the timing of oligodendrocyte
differentiation. Journal of Cell Biology 109,
3411-7.
Hebert, J.M.,
Rosenquist, T., Gotz, J., and Martin, G.R. (1994). FGF5 as a
regulator of the hair growth cycle: evidence from targeted and
spontaneous mutations. Cell 78, 1017-25.
Heldin, C.-H.
and Westermark, B. (1989). Platelet-derived growth factor: three
isoforms and two receptor types. Trends in Genetics 5,
108-11.
Hui, C.C. and
Joyner, A.L. (1993). A mouse model of greig cephalopolysyndactyly
syndrome: the extra-toes mutation contains an intragenic deletion
of the Gli3 gene. Nature Genetics 3, 241-6.
[published erratum in Nature Genetics (1998) 19,
404.]
Ibarrola, N.,
Mayer-Pröschel, M., Rodriguez-Peña, A., and Noble, M. (1996).
Evidence for the existence of at least two timing mechanisms that
contribute to oligodendrocyte generation in vitro. Developmental
Biology 180, 1-21.
Ibarrola, N.
and Rodriguez-Peña, A. (1997). Hypothyroidism coordinately and
transiently affects myelin protein gene expression in most rat
brain regions during postnatal development. Brain Research
752, 285-93.
Ikenaka, K.,
Kagawa, T., and Mikoshiba, K. (1993). Selective expression of
DM-20, and alternatively spliced myelin proteolipid protein gene
product, in developing nervous system and in nonglial cells. Journal
of Neurochemistry 58, 2248-53.
Jensen, A.M.
and Wallace, V.A. (1997). Expression of Sonic hedgehog and its
putative role as a precursor cell mitogen in the developing mouse
retina. Development 124, 363-71.
Kahn, M.A.,
Kumar, S., Liebl, D., Chang, R., Parada, L.F., and de Vellis, J.
(1999). Mice lacking NT-3, and its receptor TrkC, exhibit
profound deficiencies in CNS glial cells. Glia 26,
153-65.
Kalman, M.
and Tuba, A. (1998). Differences in myelination between spinal
cord and corticular tissue transplanted intraocularly in rats. International
Journal of Developmental Neuroscience 16, 115-21.
Kalyani, A.,
Hobson, K., and Rao, M.S. (1997). Neuroepithelial stem cells from
the embryonic spinal cord: isolation, characterization, and
clonal analysis. Developmental Biology 186, 202-23.
Kinzler,
K.W., Bigner, S.H., Bigner, D.D., Trent, J.M., Law, M.L.,
O'Brien, S.J., Wong, A.J., and Vogelstein, B. (1987).
Identification of an amplified, highly expressed gene in a human
glioma. Science 236, 70-3.
Kondo, T. and
Raff, M. (2000). The ID4 HLH protein and the timing of
oligodendrocyte differentiation. EMBO Journal 19,
1998-2007.
Kramer, R.,
Bucay, N., Kane, D.J., Martin, L.E., Tarpley, J.E., and Theill,
L.E. (1996). Neuregulins with an Ig-like domain are essential for
mouse myocardial and neuronal development. Proceedings of the
National Academy of Science USA 93, 4833-8.
Kuhlbrodt,
K., Herbarth, B., Sock, E., Hermans-Borgmeyer, I., and Wegner, M.
(1998). Sox10, a novel transcriptional modulator in glial cells. Journal
of Neuroscience 18, 237-50.
Kumar, S.,
Kahn, M.A., Dinh, L., and de Vellis, J. (1998). NT-3-mediated
TrkC receptor activation promotes proliferation and cell survival
of rodent progenitor oligodendrocyte cells in vitro and in vivo. Journal
of Neuroscience Research 54, 754-65.
Leber, S.M.,
Breedlove, S.M., and Sanes, J.R. (1990). Lineage, arrangement,
and death of clonally related motoneurons in chick spinal cord. Journal
of Neuroscience 10, 2451-62.
Leber, S.M.
and Sanes, J.R. (1995). Migratory paths of neurons and glia in
the embryonic chick spinal cord. Journal of Neuroscience 15,
1236-48.
Lee, J.C.,
Mayer-Proschel, M., and Rao, M.S. (2000). Gliogenesis in the
central nervous system. Glia 30, 105-21.
Lee, K.F.,
Simon, H., Chen, H., Bates, B., Hung, M.C., and Hauser, C.
(1995). Requirement for neuregulin receptor erbB2 in neural and
cardiac development. Nature 378, 394-8.
Lee, K.J. and
Jessell, T.M. (1999). The specification of dorsal cell fates in
the vertebrate central nervous system. Annual Reviews of
Neuroscience 22, 261-94.
Lemke, G.E.
and Brockes, J.P. (1984). Identification and purification of
glial growth factor. Journal of Neuroscience 4,
75-83.
Levine, E.M.,
Roelink, H., Turner, J., and Reh, T.A. (1997). Sonic hedgehog
promotes rod photoreceptor differentiation in mammalian retinal
cells in vitro. Journal of Neuroscience 17,
6277-88.
Levine, J.M.
and Stallcup, W.B. (1987). Plasticity of developing cerebellar
cells in vitro studied with antibodies against the NG2 antigen. Journal
of Neuroscience 7, 2721-31.
Levine, S.M.
and Goldman, J.E. (1988). Spatial and temporal patterns of
oligodendrocyte differentiation in rat cerebrum and cerebellum. Journal
of Comparative Neurology 277, 441-55.
Levison, S.W.
and Goldman, J.E. (1993). Both oligodendrocytes and astrocytes
develop from progenitors in the subventricular zone of postnatal
rat forebrain. Neuron 10, 201-12.
Levison, S.W.
and Goldman, J.E. (1997). Multipotential and lineage restricted
precursors coexist in the mammalian perinatal subventricular
zone. Journal of Neuroscience Research 48, 83-94.
Li, X.,
Pontén, A., Aase, K., Karlsson, L., Abramsson, A., Uutelo, M.,
Bäckström, G., Hellström, M., Boström, H., Li, H., Soriano,
P., Betsholtz, C., Heldin, C.-H., Alitalo, K., and Eriksson, U.
(2000). PDGF-C is a new protease-activated ligand for the PDGF a -receptor. Nature
Cell Biology 2, 302-9.
Liem, K.F.J.,
Tremml, G., Roelink, H., and Jessell, T.M. (1995). Dorsal
differentiation of neural plate cells induced by BMP-mediated
signals from epidermal ectoderm. Cell 82, 969-79.
Lu, Q.R.,
Yuk, D., Alberta, J.A., Zhu, Z., Pawlitzky, I., Chan, J.,
McMahon, A., Stiles, C.D., and Rowitch, D.H. (2000). Sonic
hedgehog-regulated oligodendrocyte lineage genes encoding bHLH
proteins in the mammalian central nervous system. Neuron 25,
317-29.
Mabie, P.C.,
Mehler, M.F., and Kessler, J.A. (1999). Multiple roles of bone
morphogenetic protein signaling in the regulation of cortical
cell number and phenotype. Journal of Neuroscience 19,
7077-88.
Mansour,
S.L., Goddard, J.M., and Capecchi, M.R. (1993). Mice homozygous
for a targeted disruption of the proto-oncogene int-2 have
developmental defects in the tail and inner ear. Development
117, 13-28.
Marchionni,
M.A., Goodearl, A.D., Chen, M.S., Bermingham-McDonogh, O., Kirk,
C., Hendricks, M., Danehy, F., Misumi, D., Sudhalter, J., and
Kobayashi, K. (1993). Glial growth factors are alternatively
spliced erbB2 ligands expressed in the nervous system. Nature
362, 312-8.
Marta, C.B.,
Adamo, A.M., Soto, E.F., and Pasquini, J.M. (1998). Sustained
neonatal hyperthyroidism in the rat affects myelination in the
central nervous system. Journal of Neuroscience Research 53,
251-9.
Matise, M.P.,
Epstein, D.J., Park, H.L., Platt, K.A., and Joyner, A.L. (1998).
Gli2 is required for induction of floor plate and adjacent cells,
but not most ventral neurons in the mouse central nervous system.
Development 125, 2759-70.
Mayer, M.,
Bhakoo, K., and Noble, M. (1994). Ciliary neurotrophic factor and
leukemia inhibitory factor promote the generation, maturation and
survival of oligodendrocyte progenitors in vitro. Development
120, 143-53.
McKinnon,
R.D., Matsui, T., Dubois-Dalcq, M., and Aaronson, S.A. (1990).
FGF modulates the PDGF-driven pathway of oligodendrocyte
development. Neuron 5, 603-14.
McMorris,
F.A. and Dubois-Dalcq, M. (1988). Insulin-like growth factor I
promotes cell proliferation and oligodendroglial commitment in
rat glial progenitor cells developing in vitro. Journal of
Neuroscience Research 21, 199-209.
McMorris,
F.A., Smith, T.M., DeSalvo, S., and Furlanetto, R.W. (1986).
Insulin-like growth factor I/somatomedin C: a potent inducer
of oligodendrocyte development. Proceedings of the National
Academy of Sciences USA 83, 822-6.
Mehler, M.F.,
Mabie, P.C., Zhu, G., Gokhan, S., and Kessler, J.A. (2000).
Developmental changes in progenitor cell responsiveness to bone
morphogenetic proteins differentially modulate progressive CNS
lineage fate. Developmental Neuroscience 22, 74-85.
Meyer, D. and
Birchmeier, C. (1995). Multiple essential functions of neuregulin
in development Nature 378, 386-90. [published
erratum in Nature (1995) 378, 753.]
Meyer, D.,
Yamaai, T., Garratt, A., Riethmacher-Sonnenberg, E., Kane, D.,
Theill, L.E., and Birchmeier, C. (1997). Isoform-specific
expression and function of neuregulin. Development 124,
3575-86.
Meyers, E.N.,
Lewandoski, M., and Martin, G.R. (1998). An Fgf8 mutant allelic
series generated by Cre- and Flp-mediated recombination. Nature
Genetics 18, 136-41.
Miller, D.L.,
Ortega, S., Bashayan, O., Basch, R., and Basilico, C. (2000).
Compensation by fibroblast growth factor 1 (FGF1) does not
account for the mild phenotypic defects observed in FGF2 null
mice. Molecular Cell Biology 20, 2260-8.
Miller, R.H.
(1996). Oligodendrocyte origins. Trends in Neurosciences 19,
92-6.
Min, H.,
Danilenko, D.M., Scully, S.A., Bolon, B., Ring, B.D., Tarpley,
J.E., DeRose, M., and Simonet, W.S. (1998). Fgf-10 is required
for both limb and lung development and exhibits striking
functional similarity to Drosophila branchless. Genes and
Development 12, 3156-61.
Mudhar, H.S.,
Pollock, R.A., Wang, C., Stiles, C.D., and Richardson, W.D.
(1993). PDGF and its receptors in the developing rodent retina
and optic nerve. Development 118, 539-52.
Nishiyama,
A., Lin, X.-H., Giese, N., Heldin, C.-H., and Stallcup, W.B.
(1996). Co-localization of NG2 proteoglycan and PDGF ? receptor
on O2A progenitor cells in the developing rat brain. Journal
of Neuroscience Research 43, 299-314.
Noble, M.,
Murray, K., Stroobant, P., Waterfield, M.D., and Riddle, P.
(1988). Platelet-derived growth factor promotes division and
motility and inhibits premature differentiation of the
oligodendrocyte/type-2 astrocyte progenitor cell. Nature 333,
560-2.
Nornes, H.O.
and Das, G.D. (1974). Temporal pattern of neurogenesis in spinal
cord of rat. I. An autoradiographic study- Time and sites of
origin and migration and settling patterns of neuroblasts. Brain
Research 73, 121-38.
Ono, K.,
Bansal, R., Payne, J., Rutishauser, U., and Miller, R.H. (1995).
Early development and dispersal of oligodendrocyte precursors in
the embryonic chick spinal cord. Development 121,
1743-54.
Ono, K.,
Yasui, Y., Rutishauser, U., and Miller, R.H. (1997). Focal
ventricular origin and migration of oligodendrocyte precursors
into the chick optic nerve. Neuron 19, 283-92.
Orentas, D.M.
and Miller, R.H. (1996). The origin of spinal cord
oligodendrocytes is dependent on local influences from the
notochord. Developmental Biology 177, 43-53.
Orentas,
D.M., Hayes, J.E., Dyer, K.L., and Miller, R.H. (1999). Sonic
hedgehog signaling is required during the appearance of spinal
cord oligodendrocyte precursors. Development 126,
2419-29.
Orr-Urtreger,
A., Trakhtenbrot, L., Ben-Levy, R., Wen, D., Rechavi, G., Lonai,
P., and Yarden, Y. (1993). Neural expression and chromosomal
mapping of Neu differentiation factor to 8p12-p21. Proceedings
of the National Academy of Sciences USA 90, 1867-71.
Ortega, S.,
Ittmann, M., Tsang, S.H., Ehrlich, M., and Basilico, C. (1998).
Neuronal defects and delayed wound healing in mice lacking
fibroblast growth factor 2. Proceedings of the National
Academy of Sciences USA 95, 5672-7.
Parnavelas,
J.G., Barfield, J.A., Franke, E., and Luskin, M.B. (1991).
Separate progenitor cells give rise to pyramidal and nonpyramidal
neurons in the rat telencephalon. Cerebral Cortex 1,
463-8.
Perez, V.E.,
Olivier, C., Spassky, N., Poncet, C., Cochard, P., Zalc, B.,
Thomas, J.L., and Martinez, S. (1999). Early specification of
oligodendrocytes in the chick embryonic brain. Developmental
Biology 216, 98-113.
Peyron, F.,
Timsit, S., Thomas, J.-L., Kagawa, T., Ikenaka, K., and Zalc, B.
(1997). In situ expression of PLP/DM-20, MBP, and CNP during
embryonic and postnatal development of the jimpy mutant and of
transgenic mice overexpressing PLP. Journal of Neuroscience
Research 50, 190-201.
Pfeiffer,
S.E., Warrington, A.E., and Bansal, R. (1994). The
oligodendrocyte and its many cellular processes. Trends in
Cell Biology 3, 191-7.
Poncet, C.,
Soula, C., Trousse, F., Kan, P., Hirsinger, E., Pourquié, O.,
Duprat, A.-M., and Cochard, P. (1996). Induction of
oligodendrocyte precursors in the trunk neural tube by
ventralizing signals: effects of notochord and floor plate
grafts, and of sonic hedgehog. Mechanisms of Development 60,
13-32.
Pringle, N.P.
and Richardson, W.D. (1993). A singularity of PDGF alpha-receptor
expression in the dorsoventral axis of the neural tube may define
the origin of the oligodendrocyte lineage. Development 117,
525-33.
Pringle,
N.P., Yu, W.-P., Guthrie, S., Roelink, H., Lumsden, A., Peterson,
A.C., and Richardson, W.D. (1996). Determination of
neuroepithelial cell fate: induction of the oligodendrocyte
lineage by ventral midline cells and Sonic hedgehog. Developmental
Biology 177, 30-42.
Pringle,
N.P., Guthrie, S., Lumsden, A., and Richardson, W.D. (1998).
Dorsal spinal cord neuroepithelium generates astrocytes but not
oligodendrocytes. Neuron 20, 883-93.
Pringle,
N.P., Yu, W.-P., Colvin, J.S., Ornitz, D.M., and Richardson, W.D.
(2000). FGFR3 in astrocytes and their precursors. In preparation.
Raabe, T.D.,
Suy, S., Welcher, A., and DeVries, G.H. (1997). Effect of neu
differentiation factor isoforms on neonatal oligodendrocyte
function. Journal of Neuroscience Research 50,
755-68.
Raff, M.C.,
Abney, E., Brockes, J.P., and Hornby-Smith, A. (1978). Schwann
cell growth factors. Cell 15, 813-22.
Raff, M.C.,
Miller, R.H., and Noble, M. (1983). A glial progenitor cell that
develops in vitro into an astrocyte or an oligodendrocyte
depending on the culture medium. Nature 303, 390-6.
Raff, M.C.,
Abney, E.R., and Fok-Seang, J. (1985). Reconstitution of a
developmental clock in vitro: a critical role for astrocytes in
the timing of oligodendrocyte differentiation. Cell 42,
61-9.
Raff, M.C.,
Lillien, L.E., Richardson, W.D., Burne, J.F., and Noble, M.
(1988). Platelet-derived growth factor from astrocytes drives the
clock that times oligodendrocyte development in culture. Nature
333, 562-5.
Raff, M.C.,
Barres, B.A., Burne, J.F., Coles, H.S., Ishazaki, Y., and
Jacobson, M.D. (1993). Programmed cell death and the control of
cell survival: lessons from the nervous system. Science 262,
695-700.
Rao, M.S.,
Noble, M., and Mayer-Proschel, M. (1998). A tripotential glial
precursor cell is present in the developing spinal cord. Proceedings
of the National Academy of Sciences USA 95, 3996-4001.
Reynolds, R.
and Wilkin, G.P. (1988). Development of macroglial cells in rat
cerebellum. II. An in situ immunohistochemical study of
oligodendroglial lineage from precursor to mature myelinating
cell. Development 102, 409-25.
Richardson,
W.D., Pringle, N., Mosley, M.J., Westermark, B., and
Dubois-Dalcq, M. (1988). A role for platelet-derived growth
factor in normal gliogenesis in the central nervous system. Cell
53, 309-19.
Richardson,
W.D., Smith, H.K., Sun, T., Pringle, N.P., Hall, A., and
Woodruff, R. (2000). Oligodendrocyte lineage and the motor neuron
connection. Glia 12, 136-42.
Riethmacher,
D., Sonnenberg-Riethmacher, E., Brinkmann, V., Yamaai, T., Lewin,
G.R., and Birchmeier, C. (1997). Severe neuropathies in mice with
targeted mutations in the ErbB3 receptor. Nature 389,
725-30.
Robinson, S.
and Miller, R.H. (1996). Environmental enhancement of growth
factor-mediated oligodendrocyte precursor proliferation. Molecular
and Cellular Neuroscience 8, 38-52.
Robinson, S.,
Tani, M., Strieter, R.M., Ransohoff, R.M., and Miller, R.H.
(1998). The chemokine growth-regulated oncogene-alpha promotes
spinal cord oligodendrocyte precursor proliferation. Journal
of Neuroscience 18, 10457-63.
Rodriguez-Peña,
A. (2000). Oligodendrocyte development and thyroid hormone. Journal
of Neurobiology 40, 497-512.
Roelink, H.,
Augsburger, A., Heemskerk, J., Korzh, V., Norlin, S., Ruiz i
Altaba, A., Tanabe, Y., Placzek, M., Edlund, T., Jessell, T.M.,
and Dodd, J. (1994). Floor plate and motor neuron induction by
vhh-1, a vertebrate homolog of hedgehog expressed by the
notochord. Cell 76, 761-75.
Rogister, B.,
Ben-Hur, T., and Dubois-Dalcq, M. (1999). From neural stem cells
to myelinating oligodendrocytes. Molecular and Cellular
Neuroscience 14, 287-300.
Rowitch,
D.H., Jacques, B., Lee, S.M., Flax, J.D., Snyder, E.Y., and
McMahon, A.P. (1999). Sonic hedgehog regulates proliferation and
inhibits differentiation of CNS precursor cells. Journal of
Neuroscience 19, 8954-65.
Ruiz i
Altaba, A. (1998). Combinatorial Gli gene function in floor plate
and neuronal inductions by Sonic hedgehog. Development 125,
2203-12.
Ruiz i
Altaba, A. (1999). Gli proteins and Hedgehog signaling:
development and cancer. Trends in Genetics 15,
418-25.
Sekine, K.,
Ohuchi, H., Fujiwara, M., Yamasaki, M., Yoshizawa, T., Sato, T.,
Yagishita, N., Matsui, D., Koga, Y., Itoh, N., and Kato, S.
(1999). Fgf10 is essential for limb and lung formation. Nature
Genetics 21, 138-141.
Shi, J.,
Marinovich, A., and Barres, B.A. (1998). Purification and
characterization of adult oligodendrocyte precursor cells from
the rat optic nerve. Journal of Neuroscience 18,
4627-36.
Spassky, N.,
Goujet-Zalc, C., Parmantier, E., Olivier, C., Martinez, S.,
Ivanova, A., Ikenaka, K., Macklin, W., Cerruti, I., Zalc, B., and
Thomas, J.-L. (1998). Multiple restricted origin of
oligodendrocytes. Journal of Neuroscience 18,
8331-43.
Spassky, N.,
Olivier, C., Perez-Villegas, E., Goujet-Zalc, C., Martinez, S.,
Thomas, J., and Zalc, B. (2000). Single or multiple
oligodendroglial lineages: a controversy. Glia 29,
143-8.
Stallcup,
W.B. and Beasley, L. (1987). Bipotential glial progenitor cells
of the optic nerve express the NG2 proteoglycan. Journal of
Neuroscience 7, 2737-44.
Stone, D.M.,
Hynes, M., Armanini, M., Swanson, T.A., Gu, Q., Johnson, R.L.,
Scott, M.P., Pennica, D., Goddard, A., Phillips, H., Noll, M.,
Hooper, J.E., de Sauvage, F., and Rosenthal, A. (1996). The
tumour-suppressor gene patched encodes a candidate receptor for
Sonic hedgehog. Nature 384, 129-34.
Sun, T.,
Pringle, N.P., Hardy, A.P., Richardson, W.D., and Smith, H.K.
(1998). Pax6 inluences the time and site of origin of glial
precursors in the ventral neural tube. Molecular and Cellular
Neuroscience 12, 228-39.
Sussel, L.,
Marin, O., Kimura, S., and Rubenstein, J.L. (1999). Loss of
Nkx2.1 homeobox gene function results in a ventral to dorsal
molecular respecification within the basal telencephalon:
evidence for a transformation of the pallidum into the striatum. Development
126, 3359-70.
Tanabe, Y.
and Jessell, T.M. (1996). Diversity and pattern in the developing
spinal cord. Science 274, 1115-23.
Tang, D.G.,
Tokumoto, Y.M., and Raff, M.C. (2000). Long-term culture of
purified postnatal oligodendrocyte precursor cells: evidence for
an intrinsic maturation program that plays out over months. Journal
of Cell Biology 148, 971-84.
Temple, S.
and Raff, M.C. (1985). Differentiation of a bipotential glial
progenitor cell in single cell microculture. Nature 313,
223-5.
Temple, S.
and Raff, M.C. (1986). Clonal analysis of oligodendrocyte
development in culture: evidence for a developmental clock that
counts cell divisions. Cell 44, 773-9.
Thomas, J.,
Spassky, N., Perez, V.E., Olivier, C., Cobos, I., Goujet-Zalc,
C., Martinez, S., and Zalc, B. (2000). Spatiotemporal development
of oligodendrocytes in the embryonic brain. Journal of
Neuroscience Research 59, 471-6.
Timsit, S.G.,
Bally-Cuif, L., Colman, D.R., and Zalc, B. (1992). DM-20 mRNA is
expressed during the embryonic development of the nervous system
of the mouse. Journal of Neurochemistry 58, 1172-5.
Timsit, S.,
Martinez, S., Allinquant, B., Peyron, F., Puelles, L., and Zalc,
B. (1995). Oligodendrocytes originate in a restricted zone of the
embryonic ventral neural tube defined by DM-20 mRNA expression. Journal
of Neuroscience 15, 1012-24.
van
Heyningen, P., Calver, A.R., Woodruff, R., and Richardson, W.D.
(2000). Evidence that glial progenitor cell number in the
embryonic spinal cord is determined by competition for limiting
amounts of cell-extrinsic mitogens including PDGF. In
preparation.
Vartanian,
T., Corfas, G., Li, Y., Fischbach, G.D., and Stefansson, K.
(1994). A role for the acetylcholine receptor-inducing protein
ARIA in oligodendrocyte development. Proceedings of the
National Academy of Sciences USA 91, 11626-30.
Vartanian,
T., Fischbach, G., and Miller, R. (1999). Failure of spinal cord
oligodendrocyte development in mice lacking neuregulin. Proceedings
of the National Academy of Sciences USA 96, 731-5.
Vitry, S.,
Avellana-Adalid, V., Hardy, R., Lachapelle, F., and Baron-Van,
E.A. (1999). Mouse oligospheres: from pre-progenitors to
functional oligodendrocytes. Journal of Neuroscience Research
58, 735-51.
Vos, J.P.,
Gard, A.L., and Pfeiffer, S.E. (1996). Regulation of
oligodendrocyte cell survival and differentiation by ciliary
neurotrophic factor, leukemia inhibitory factor, oncostatin M,
and interleukin-6. Perspectives in Developmental Neurobiology
4, 39-52.
Wallace, V.A.
and Raff, M.C. (1999). A role for Sonic hedgehog in
axon-to-astrocyte signalling in the rodent optic nerve. Development
126, 2901-9.
Walters, S.N.
and Morell, P. (1981). Effects of altered thyroid states on
myelinogenesis. Journal of Neurochemistry 36,
1792-801.
Wang, S.,
Sdrulla, A.D., diSibio, G., Bush, G., Nofziger, D., Hicks.C.,
Weinmaster, G., and Barres, B.A. (1998). Notch receptor
activation inhibits oligodendrocyte differentiation. Neuron
21, 63-75.
Wang, Y.,
Spatz, M.K., Kannan, K., Hayk, H., Avivi, A., Gorivodsky, M.,
Pines, M., Yayon, A., Lonai, P., and Givol, D. (1999). A mouse
model for achondroplasia produced by targeting fibroblast growth
factor receptor 3. Proceedings of the National Academy of
Sciences USA 96, 4455-60.
Warf, B.C.,
Fok-Seang, J., and Miller, R.H. (1991). Evidence for the ventral
origin of oligodendrocyte precursors in the rat spinal cord. Journal
of Neuroscience 11, 2477-88.
Williams,
B.P. and Price, J. (1995). Evidence for multiple precursor cell
types in the embryonic rat cerebral cortex. Neuron 14,
1181-8.
Williams,
B.P., Read, J., and Price, J. (1991). The generation of neurons
and oligodendrocytes from a common precursor cell. Neuron 7,
685-93.
Woldeyesus,
M.T., Britsch, S., Riethmacher, D., Xu, L.,
Sonnenberg-Riethmacher, E., Abou-Rebyeh, F., Harvey, R., Caroni,
P., and Birchmeier, C. (1999). Peripheral nervous system defects
in erbB2 mutants following genetic rescue of heart development. Genes
and Development 13, 2538-48.
Wood, P.M.
and Bunge, R.P. (1975). Evidence that sensory axons are mitogenic
for Schwann cells. Nature 256, 662-4.
Tekki-Kessaris,
N., Woodruff, R., Hall, A.C., Pringle, N.P., Kimura, S., Stiles,
C.D., Rowitch, D., and Richardson, W.D. (2000). Ventral origin of
oligodendrocytes in the telencephalon. Submitted
Wu, Q.,
Miller, R.H., Ransohoff, R.M., Robinson, S., Bu, J., and
Nishiyama, A. (2000). Elevated levels of the chemokine GRO-1
correlate with elevated oligodendrocyte progenitor proliferation
in the jimpy mutant. Journal of Neuroscience 20,
2609-17.
Yamaguchi,
T.P., Harpal, K., Henkemeyer, M., and Rossant, J. (1994). fgfr-1
is required for embryonic growth and mesodermal patterning during
mouse gastrulation. Genes and Development 8,
3032-44.
Ye, P.,
Carson, J., and D'Ercole, A.J. (1995). In vivo actions of
insulin-like growth factor-I (IGF-I) on brain myelination:
studies of IGF-I and IGF binding protein-1 (IGFBP-1) transgenic
mice. Journal of Neuroscience 15, 7344-56.
Yeh, H.-J.,
Ruit, K.G., Wang, Y.-X., Parks, W.C., Snider, W.D., and Deuel,
T.F. (1991). PDGF A-chain gene is expressed by mammalian neurons
during development and in maturity. Cell 64,
209-16.
Yu, W.-P.,
Collarini, E.J., Pringle, N.P., and Richardson, W.D. (1994).
Embryonic expression of myelin genes: evidence for a focal source
of oligodendrocyte precursors in the ventricular zone of the
neural tube. Neuron 12, 1353-62.
Zerlin, M.,
Levison, S.W., and Goldman, J.E. (1995). Early patterns of
migration, morphogenesis and intermediate filament expression of
subventricular zone cells in the postnatal rat forebrain. Journal
of Neuroscience 15, 7238-49.
Zhang, H. and
Miller, R.H. (1995). Asynchronous differentiation of clonally
related spinal cord oligodendrocytes. Molecular and Cellular
Neuroscience 6, 16-31.
Zhou, Q.,
Wang, S., and Anderson, D.J. (2000). Identification of a novel
family of oligodendrocyte lineage-specific basic helix-loop-helix
transcription factors. Neuron 25, 331-43.
Zhu, G.,
Mehler, M.F., Zhao, J., Yu, Y.S., and Kessler, J.A. (1999). Sonic
hedgehog and BMP2 exert opposing actions on proliferation and
differentiation of embryonic neural progenitor cells. Developmental
Biology 215, 118-29.
back to index
back to top
 Figure 1 Appearance and spread of PDGFRa +
oligodendrocyte progenitors in the embryonic mouse spinal cord
and forebrain, visualized by in situ hybridization. In the spinal
cord the first progenitors appear in the ventral VZ on E13
(arrow), then they proliferate and migrate away from the midline.
Within a day-and-a-half (E14.5) they spread through most of the
cord. The equivalent ages in rat are E14 to E15.5 and in chick,
E7 to E9. In the forebrain (lower left), a cluster of PDGFRa +cells
appears in the ventral diencephalon (anterior hypothalamic
neuroepithelium) before E13 (arrow). These cells subsequently
proliferate and migrate into the dorsal forebrain including the
developing cortex (Cx) before birth (not shown). At higher
magnification (lower right) it can be seen that the migratory
cells are intensely labelled PDGFRa +
cells (arrows) that develop within the initial cluster of less
strongly labelled neuroepithelial cells (asterisk). MGE, medial
ganglionic eminence; LGE, lateral ganglionic eminence. back to index
Figure 1 Appearance and spread of PDGFRa +
oligodendrocyte progenitors in the embryonic mouse spinal cord
and forebrain, visualized by in situ hybridization. In the spinal
cord the first progenitors appear in the ventral VZ on E13
(arrow), then they proliferate and migrate away from the midline.
Within a day-and-a-half (E14.5) they spread through most of the
cord. The equivalent ages in rat are E14 to E15.5 and in chick,
E7 to E9. In the forebrain (lower left), a cluster of PDGFRa +cells
appears in the ventral diencephalon (anterior hypothalamic
neuroepithelium) before E13 (arrow). These cells subsequently
proliferate and migrate into the dorsal forebrain including the
developing cortex (Cx) before birth (not shown). At higher
magnification (lower right) it can be seen that the migratory
cells are intensely labelled PDGFRa +
cells (arrows) that develop within the initial cluster of less
strongly labelled neuroepithelial cells (asterisk). MGE, medial
ganglionic eminence; LGE, lateral ganglionic eminence. back to index


